Using tissue oxygen levels to assess ex vivo brain slice quality
Measurement of tissue oxygen partial pressure provides an easy, quick and reliable method for screening and controlling for variation in ex vivo acute brain slice viability.
Introduction
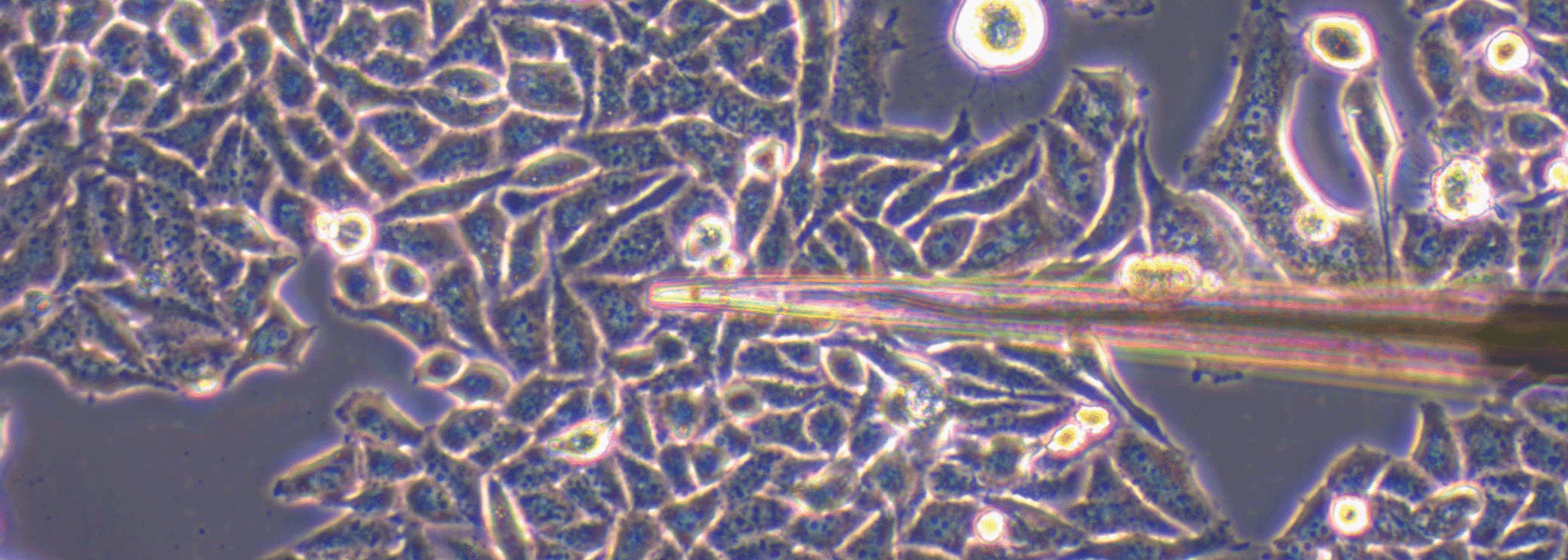
The acute ex vivo brain slice model is a versatile tool for investigating neurophysiological processes, allowing experimental conditions to be tightly controlled. However, the utility of the method can be compromised by variation in tissue quality, which can be difficult to reliably assess using simple electrophysiological approaches.
We all know that animals rely on oxygen to fuel the production of energy to sustain living tissue. Intuitively, it is also well understood that the more active the tissue, the greater the oxygen requirement to maintain that activity. In this study, we utilized this simple relationship to develop a straightforward method for reliably assessing the quality of acute tissue slices, following the rationale that healthier tissue will consume more oxygen.
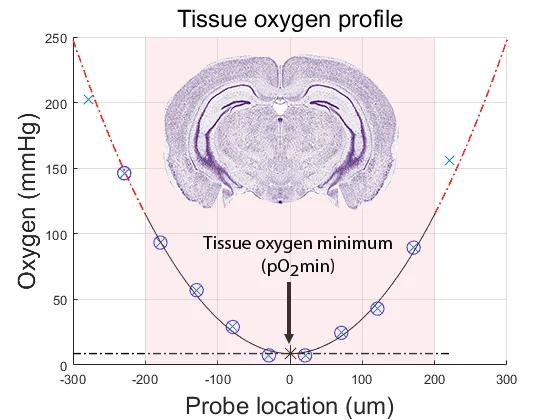
If one knows the tissue oxygen solubility and diffusion characteristics (the “Krogh” coefficient), oxygen consumption can be accurately quantified from the curvature of the tissue depth versus oxygen partial pressure profile. However, accurate tissue oxygen profiling is time consuming and requires offline calculation. We hypothesized that the tissue oxygen minimum level (pO2min) would provide a more accessible method.
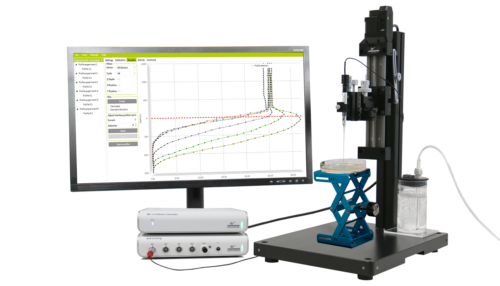
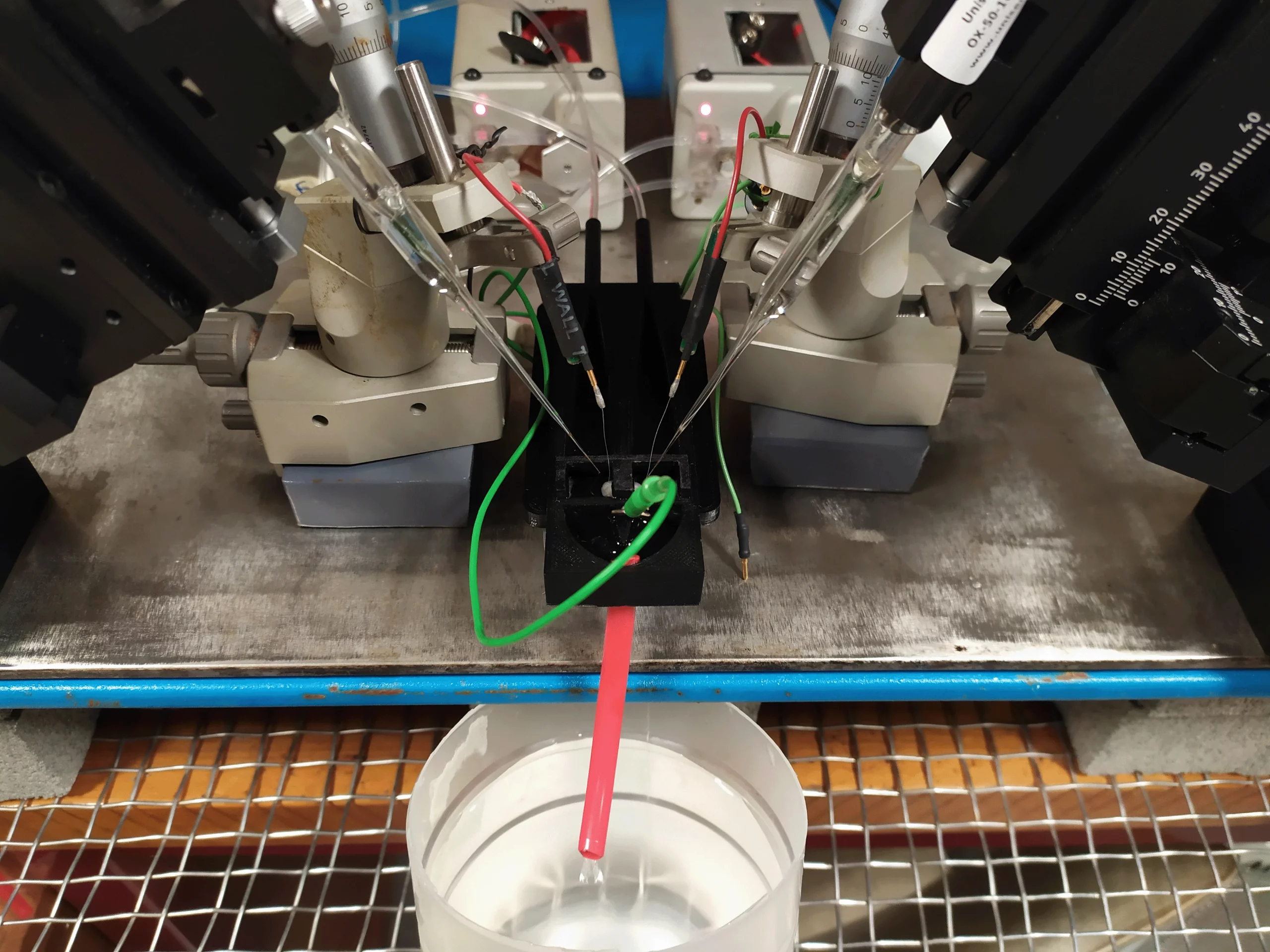
Laboratory setup
The appeal of this method is its simplicity. Using a Unisense oxygen electrode attached to a standard micromanipulator, the electrode is advanced through the tissue until the oxygen partial pressure reaches pO2min. The electrodes can be retracted and advanced two or three times to confirm the true nadir in oxygen level has been identified.
Results and Conclusion
We have shown that pO2min correlates with oxygen consumption rate and tissue viability — the lower pO2min, the higher the tissue oxygen consumption and the healthier the tissue. If pO2min is zero, oxygen supply is not meeting tissue demand and the flow rate of the perfusate should be increased. The pO2min value can be used to discard unhealthy tissue or to control for variation in tissue health from one experiment to another. For the experimental parameters in this study (mouse cerebro-cortical slices perfused at room temperature at a flow rate of 6 mL/min), strong viable tissue was reflected in pO2min values of 0-50 mmHg in population-active tissue — and 150-200 mmHg in quiescent tissue.
Tissue oxygen minimum is simple to measure and provides a robust estimate of ex vivo tissue viability status. While the specific pO2min values will vary with preparation and experimental parameters, the principle is widely applicable across tissue types and perfusion platforms.
You can read more in the article by Voss et al. “Tissue oxygen partial pressure as a viability metric for ex vivo brain tissue slices”, Journal of Neuroscience Methods 396 (2023) 109932 and in the article by Steyn-Ross et al. “Determination of Krogh Coefficient for oxygen consumption measurement from thin slices of rodent cortical tissue using a Fick’s law model of diffusion”, Int. J. Mol. Sci. 2023, 24, 6450.
“The Unisense hardware, software and electrodes work flawlessly for the applications I use in the laboratory and the customer service is top-drawer”.
Dr. Logan Voss, Dept Anaesthesia, Waikato DHB
The application note was written by
Dr. Logan Voss
Department of Anaesthesia
Te Whatu Ora Health New Zealand Waikato
Hamilton 3204, New Zealand
Related publications
Application Notes
pH Microelectrode reveals distinct environment inside 3D organoid
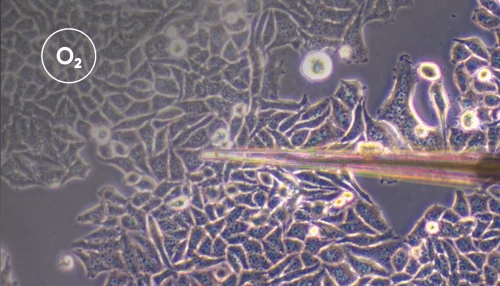
Tissue oxygen partial pressure for screening and controlling for variation in ex vivo acute brain slice viability

How nitrifying microorganisms are able to produce nitrous oxide through denitrification

Hydrogen and oxygen microsensors for direct and real-time detection of dissolved gas in photocatalytic/electrochemical studies

Learn in the laboratory - explore and confirm in the field!

Leaf gas films are hypothesized to improve internal aeration of the plant during the day

How to quantify the consumption rate of oxygen as well as the oxygen exchange rate across the water - sediment interface

Mitigation of N2O Emissions from Wastewater Biofilms
The use of oxygen microelectrodes to study nitritation biofilms with different geometries

O2 and N2O microprofiles in sputum samples from cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection
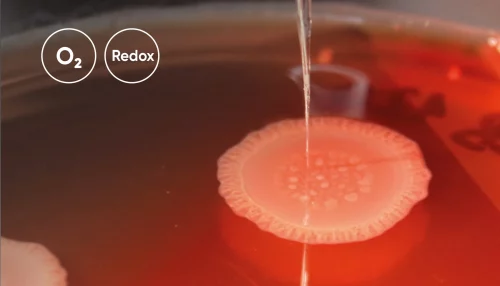
Oxygen and Redox Potential in Pseudomonas Aeruginosa Colony Biofilms

Hydrogen microsensors used to document the possible positive effect of H2-enriched drinking water as a biomedical treatment.
Analyzing gas cavity formation at implant sites

Studying oxygen conditions of in vitro cell cultures

Oxygen Microprofiles in the Retina of Antarctic Icefish