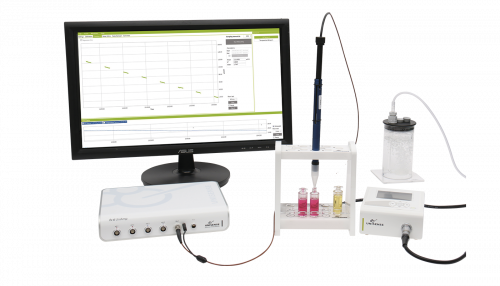
Pericellular oxygen depletion during ordinary tissue culturing, measured with oxygen microsensors
Pettersen, Erik O. et al. (2005),
Cell Proliferation,
vol. 38,
257-267
Pettersen, Erik O., Larsen, Lars Hauer, Ramsing, Niels Birger, Ebbesen, Peter (2005),
Cell Proliferation,
vol.
38,
257-267
Recent research has found important differences in oxygen tension in proximity to certain mammalian cells when grown in culture. Oxygen has a low diffusion rate through cell culture media, thus, as a result of normal respiration, a decrease in oxygen tension develops close to the cells. Therefore, for the purpose of standardization and optimization, it is important to monitor pericellular oxygen tension and cell oxygen consumption. Here, we describe an integrated oxygen microsensor and recording system that allows measurement of oxygen concentration profiles in vertical transects through a 1.6-mm deep, stagnant, medium layer covering a cell culture. The measurement set-up reveals that, when confluent, a conventional culture of adherent cells, although exposed to the constant oxygen tension of ambient air, may experience pericellular oxygen tensions below the level required to sustain full oxidative metabolism. Depletions reported are even more prominent and potentially aggravating when the cell culture is incubated at reduced oxygen tensions (down to around 4% oxygen). Our results demonstrate that, if the pericellular oxygen tension is not measured, it is impossible to relate in vitro culture results (for example, gene expression to the oxygen tension experienced by the cell), as this concentration may deviate very substantially from the oxygen concentration recorded in the gas phase. © 2005 Blackwell Publishing Ltd.
10.1111/j.1365-2184.2005.00345.x
Concentration-dependent metabolic effects of metformin in healthy and Fanconi anemia lymphoblast cells
Ravera, Silvia et al. (2018),
Journal of Cellular Physiology,
vol. 233,
1736-1751
Ravera, Silvia, Cossu, Vanessa, Tappino, Barbara, Nicchia, Elena, Dufour, Carlo, Cavani, Simona, Sciutto, Andrea, Bolognesi, Claudia, Columbaro, Marta, Degan, Paolo, Cappelli, Enrico (2018),
Journal of Cellular Physiology,
vol.
233,
1736-1751
Metformin (MET) is the drug of choice for patients with type 2 diabetes and has been proposed for use in cancer therapy and for treating other metabolic diseases. More than 14,000 studies have been published addressing the cellular mechanisms affected by MET. However, several in vitro studies have used concentrations of the drug 10–100-fold higher than the plasmatic concentration measured in patients. Here, we evaluated the biochemical, metabolic, and morphologic effects of various concentrations of MET. Moreover, we tested the effect of MET on Fanconi Anemia (FA) cells, a DNA repair genetic disease with defects in energetic and glucose metabolism, as well as on human promyelocytic leukemia (HL60) cell lines. We found that the response of wild-type cells to MET is concentration dependent. Low concentrations (15 and 150 µM) increase both oxidative phosphorylation and the oxidative stress response, acting on the AMPK/Sirt1 pathway, while the high concentration (1.5 mM) inhibits the respiratory chain, alters cell morphology, becoming toxic to the cells. In FA cells, MET was unable to correct the energetic/respiratory defect and did not improve the response to oxidative stress and DNA damage. By contrast, HL60 cells appear sensitive also at 150 μM. Our findings underline the importance of the MET concentration in evaluating the effect of this drug on cell metabolism and demonstrate that data obtained from in vitro experiments, that have used high concentrations of MET, cannot be readily translated into improving our understanding of the cellular effects of metformin when used in the clinical setting.
10.1002/jcp.26085
Divergent targets of glycolysis and oxidative phosphorylation result in additive effects of metformin and…
Marini, Cecilia et al. (2016),
Scientific Reports,
vol. 6,
1-13
Marini, Cecilia, Bianchi, Giovanna, Buschiazzo, Ambra, Ravera, Silvia, Martella, Roberto, Bottoni, Gianluca, Petretto, Andrea, Emionite, Laura, Monteverde, Elena, Capitanio, Selene, Inglese, Elvira, Fabbi, Marina, Bongioanni, Francesca, Garaboldi, Lucia, Bruzzi, Paolo, Orengo, Anna Maria, Raffaghello, Lizzia, Sambuceti, Gianmario (2016),
Scientific Reports,
vol.
6,
1-13
Emerging evidence demonstrates that targeting energy metabolism is a promising strategy to fight cancer. Here we show that combining metformin and short-term starvation markedly impairs metabolism and growth of colon and breast cancer. The impairment in glycolytic flux caused by starvation is enhanced by metformin through its interference with hexokinase II activity, as documented by measurement of 18F-fluorodeoxyglycose uptake. Oxidative phosphorylation is additively compromised by combined treatment: metformin virtually abolishes Complex I function; starvation determines an uncoupled status of OXPHOS and amplifies the activity of respiratory Complexes II and IV thus combining a massive ATP depletion with a significant increase in reactive oxygen species. More importantly, the combined treatment profoundly impairs cancer glucose metabolism and virtually abolishes lesion growth in experimental models of breast and colon carcinoma. Our results strongly suggest that energy metabolism is a promising target to reduce cancer progression.
10.1038/srep19569
Discovery of a novel glucose metabolism in cancer: The role of endoplasmic reticulum beyond glycolysis an…
Marini, Cecilia et al. (2016),
Scientific Reports,
vol. 6,
1-13
Marini, Cecilia, Ravera, Silvia, Buschiazzo, Ambra, Bianchi, Giovanna, Orengo, Anna Maria, Bruno, Silvia, Bottoni, Gianluca, Emionite, Laura, Pastorino, Fabio, Monteverde, Elena, Garaboldi, Lucia, Martella, Roberto, Salani, Barbara, Maggi, Davide, Ponzoni, Mirco, Fais, Franco, Raffaghello, Lizzia, Sambuceti, Gianmario (2016),
Scientific Reports,
vol.
6,
1-13
Cancer metabolism is characterized by an accelerated glycolytic rate facing reduced activity of oxidative phosphorylation. This "Warburg effect" represents a standard to diagnose and monitor tumor aggressiveness with 18F-fluorodeoxyglucose whose uptake is currently regarded as an accurate index of total glucose consumption. Studying cancer metabolic response to respiratory chain inhibition by metformin, we repeatedly observed a reduction of tracer uptake facing a marked increase in glucose consumption. This puzzling discordance brought us to discover that 18F-fluorodeoxyglucose preferentially accumulates within endoplasmic reticulum by exploiting the catalytic function of hexose-6-phosphate-dehydrogenase. Silencing enzyme expression and activity decreased both tracer uptake and glucose consumption, caused severe energy depletion and decreased NADPH content without altering mitochondrial function. These data document the existence of an unknown glucose metabolism triggered by hexose-6-phosphate-dehydrogenase within endoplasmic reticulum of cancer cells. Besides its basic relevance, this finding can improve clinical cancer diagnosis and might represent potential target for therapy.
10.1038/srep25092
808-Nm Photobiomodulation Affects the Viability of a Head and Neck Squamous Carcinoma Cellular Model, Act…
Ravera, Silvia et al. (2021),
Biomedicines,
vol. 9,
1-16
Ravera, Silvia, Bertola, Nadia, Pasquale, Claudio, Bruno, Silvia, Benedicenti, Stefano, Ferrando, Sara, Zekiy, Angelina, Arany, Praveen, Amaroli, Andrea (2021),
Biomedicines,
vol.
9,
1-16
Photobiomodulation (PBM) is a form of low-dose light therapy that acts through energy delivery from non-ionizing sources. During the recent two decades, there has been tremendous progress with PBM acceptance in medicine. However, PBM effects on potential stimulation of existing malignant or pre-malignant cells remain unknown. Thus, the primary endpoint was to assess the safety of PBM treatment parameters on head and neck squamous cell carcinoma (HNSCC) proliferation or survival. The secondary endpoint was to assess any putative anti-cancer effects of PBM treatments. Cell viability, energy metabolism, oxidative stress, and pro-and anti-apoptotic markers expression were investigated on a Human Head and Neck Squamous Cell Carcinoma cellular model (OHSU-974 FAcorr cell line). PBM therapy was administered through the 810 nm diode laser (GaAlAs) device (Garda Laser, 7024 Negrar, Verona, Italy) at the powers of 0, 0.25, 0.50, 0.75, 1.00, or 1.25 W in continuous wave (CW) mode for an exposure time of 60 s with a spot-size of 1 cm2 and with a distance of 1.86 cm from the cells. Results showed that 810-nm PBM affected oxidative phosphorylation in OHSU-971 FAcorr, causing a metabolic switch to anaerobic glycolysis. In addition, PBM reduced the catalase activity, determining an unbalance between oxidative stress production and the antioxidant defenses, which could stimulate the pro-apoptotic cellular pathways. Our data, at the parameters investigated, suggest the safeness of PBM as a supportive cancer therapy. Preclinical and clinical studies are necessary to confirm the in vitro evidence.
10.3390/biomedicines9111717
The human fetal and adult stem cell secretome can exert cardioprotective paracrine effects against cardio…
Villa, Federico et al. (2021),
Cancers,
vol. 13,
-
Villa, Federico, Bruno, Silvia, Costa, Ambra, Li, Mingchuan, Russo, Michele, Cimino, James, Altieri, Paola, Ruggeri, Clarissa, Gorgun, Cansu, De Biasio, Pierangela, Paladini, Dario, Coviello, Domenico, Quarto, Rodolfo, Ameri, Pietro, Ghigo, Alessandra, Ravera, Silvia, Tasso, Roberta, Bollini, Sveva (2021),
Cancers,
vol.
13,
-
Cardiovascular side effects are major shortcomings of cancer treatments causing cardiotox-icity and late-onset cardiomyopathy. While doxorubicin (Dox) has been reported as an effective chemotherapy agent, unspecific impairment in cardiomyocyte mitochondria activity has been docu-mented. We demonstrated that the human fetal amniotic fluid-stem cell (hAFS) secretome, namely the secreted paracrine factors within the hAFS-conditioned medium (hAFS-CM), exerts pro-survival effects on Dox-exposed cardiomyocytes. Here, we provide a detailed comparison of the cardiopro-tective potential of hAFS-CM over the secretome of mesenchymal stromal cells from adipose tissue (hMSC-CM). hAFS and hMSC were preconditioned under hypoxia to enrich their secretome. The cardioprotective effects of hAFS/hMSC-CM were evaluated on murine neonatal ventricular cardiomy-ocytes (mNVCM) and on their fibroblast counterpart (mNVFib), and their long-term paracrine effects were investigated in a mouse model of Dox-induced cardiomyopathy. Both secretomes significantly contributed to preserving mitochondrial metabolism within Dox-injured cardiac cells. hAFS-CM and hMSC-CM inhibited body weight loss, improved myocardial function, reduced lipid peroxidation and counteracted the impairment of mitochondrial complex I activity, oxygen consumption, and ATP synthesis induced by Dox. The hAFS and hMSC secretomes can be exploited for inhibiting cardiotoxic detrimental side effects of Dox during cancer therapy, thus ensuring cardioprotection via combinatorial paracrine therapy in association with standard oncological treatments.
10.3390/cancers13153729