
Studying the Effects of Leaf Gas Film Using Microsensors
Flooding of vegetation introduces a number of challenges to the plants. Reduced oxygen solubility combined with slower diffusion of gases restricts photosynthesis under water as O2 and CO2 exchange between the plant and the environment is limited. Some plants have developed smart ways to survive flooding. The referred two articles focus on the function of leaf gas film formed on
the superhydrophobic leaf.
There has been suggestions of improved CO2 uptake during submergence of plants with leaf gas films; however, the function of leaf gas films is not well-understood. In Pedersen et al. (2009) this function is investigated by setting up laboratory experiments and in Winkel et al. (2013) the studies are repeated in paddy field rice in the Philippines. This research summary will focus on the data the researchers obtained using microsensors in laboratory and in situ experiments.
References
A. Winkel, T. D. Colmer, A. M. Ismail, and O. Pedersen. Internal aeration of paddy field rice (Oryza sativa) during complete submergence - importance of light and floodwater O2. New Phytol 197 (4):1193-1203, 2013.
O. Pedersen, S. M. Rich, and T. D. Colmer. Surviving floods: leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice. The Plant Journal 58 (1):147-156, 2009.
Laboratory Setup
The dynamics of root pO2 in light and darkness were investigated using O2 microsensors. Four-week-old plants (Oryza sativa L.) were kept in a chamber that allowed for separate medium for root and shoot of the plant. The roots were incubated in deoxygenated medium, whereas the shoots were incubated in a medium containing 200 mmol m-3 free CO2 and O2 in air equilibrium. The chamber was covered with PVC foil to prevent possible contact between leaves and air. This setup mimicked in situ conditions and allowed for studying the internal aeration of the plant.
Unisense O2 microsensors with a tip diameter of 25 µm were connected to a Unisense amplifier, mounted on a micromanipulator and positioned into the root cortex. The effect of leaf gas film was studied by measuring root pO2 before and after brushing the leaves with a diluted Triton-X-100 solution, to remove the gas film.
Field Setup
Root pO2 of paddy field rice was measured during two days of complete submergence. Four-week-old rice plants were planted into a paddy field. Roots were exposed and the microsensors were placed 200 µm into the root tissue. Hereafter both root and microsensor were covered with soil, placing the sensor tip approx. 4 cm below soil surface. To investigate the function of the leaf gas film, leaves of selected plants had their gas film removed 3 hours prior to flooding. Light status was monitored by a weather station placed approx. 440 m from the paddy field.
Conclusion on Lab and Field Data
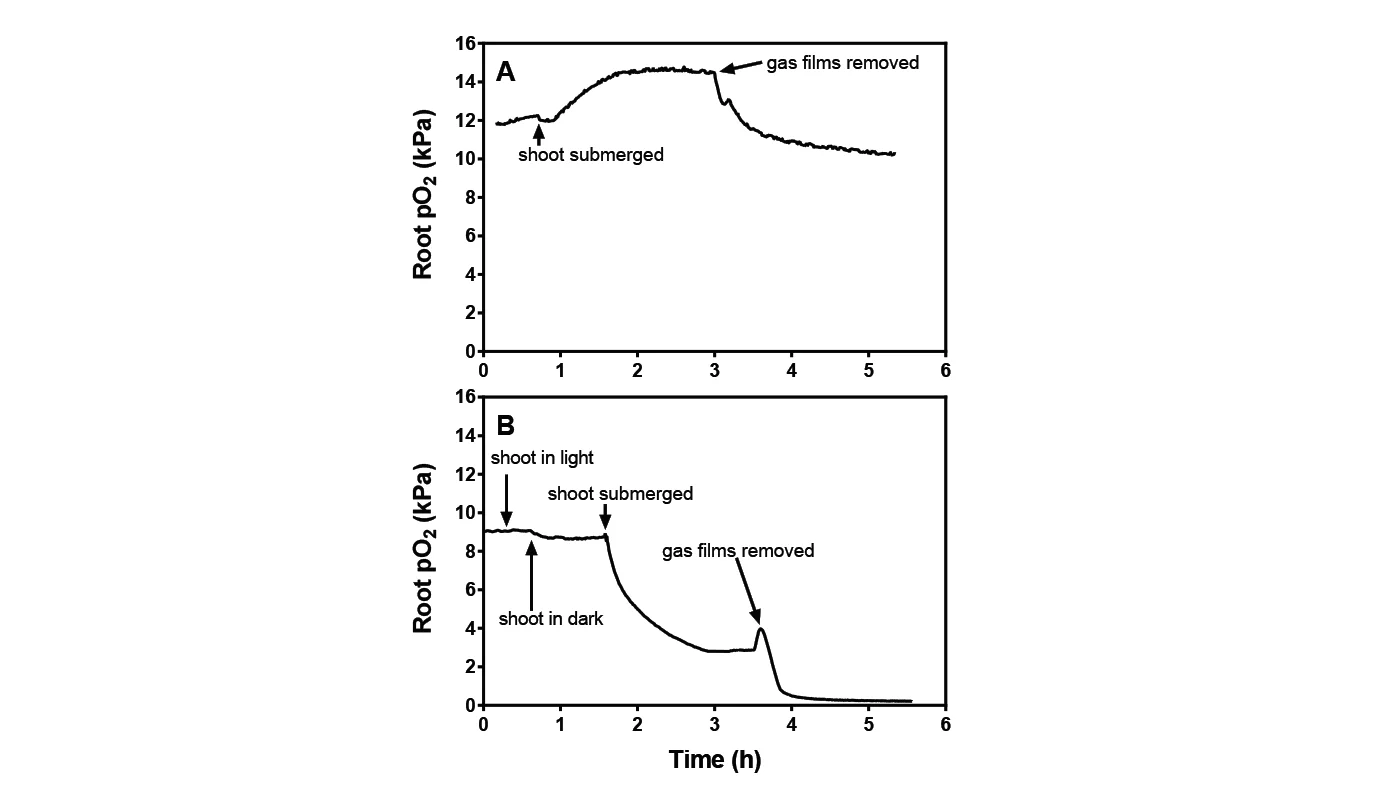
Leaf gas films are hypothesized to improve internal aeration of the plant during the day, as leaf gas films enhance CO2 uptake and thereby promote photosynthesis. This is seen as higher root pO2 in plants with intact gas film compared to plants without. The two studies described investigate gas film based on laboratory experiments and experiments in the field.
In the laboratory, root pO2 increases in light periods in submerged plants, probably due to reduced outward diffusion of photosynthetically produced O2. Removal of gas film resulted in a decrease in root pO2 to just below the initial level (see fig 1A). This can be explained by decreased O2 production by photosynthesis as a result of impeded CO2 entry. In darkness, root pO2 rapidly decreased to 25% (see fig. 1B) when submerged in light and declined to close to zero when the gas film was removed.
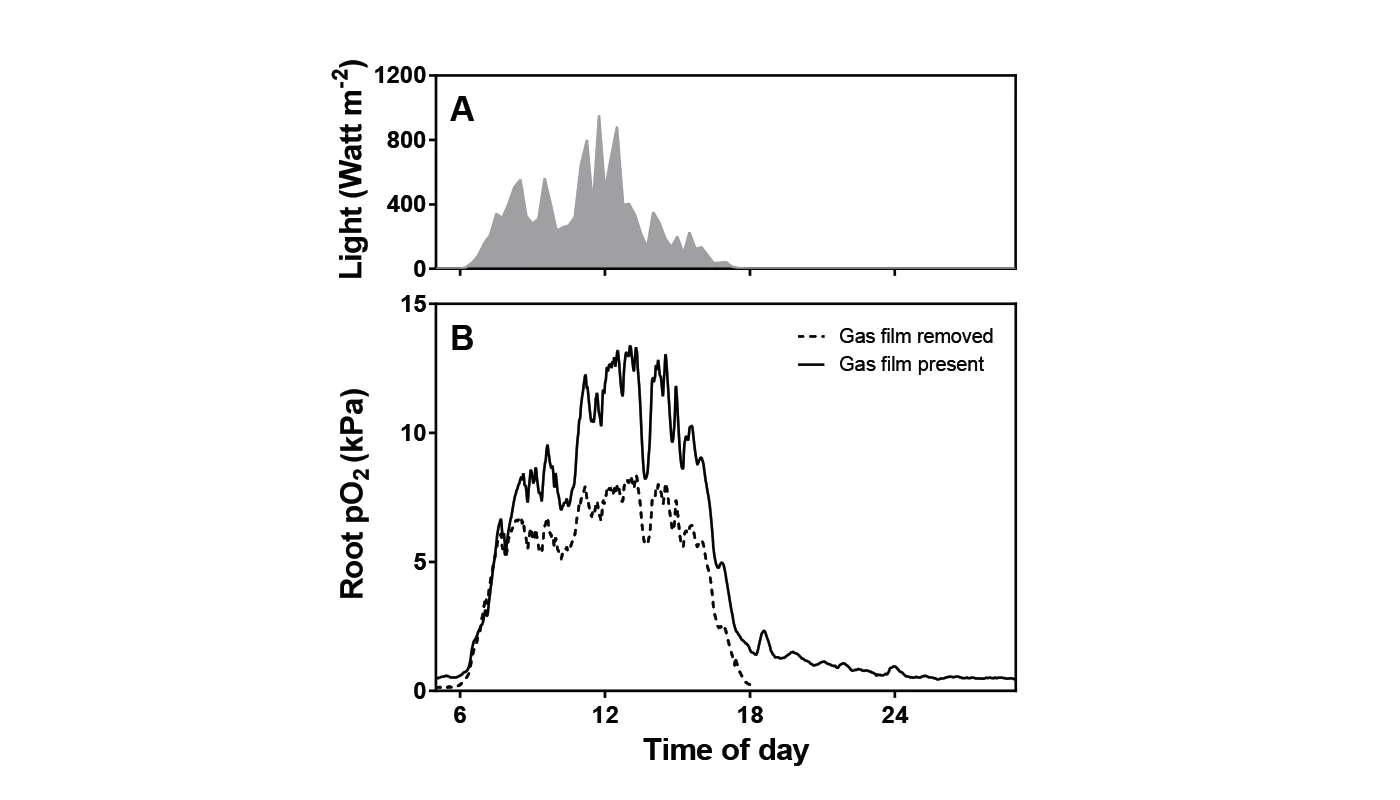
In the field, plants with intact leaf gas films had higher daytime root pO2 compared to plants without leaf gas film during the first day of measurements (fig. 2B), supporting laboratory data. The difference in root pO2 of plants with or without leaf gas film was, however, not significant on the second day (data not shown), suggesting that the leaf gas film had either been reestablished or that emerging new leaves with intact gas film had masked the effect of the gas film removal on the leaves from the day before.
Although data obtained in the field were only significant on the first day of measurement, the in situ data support the findings obtained in the laboratory experiments by showing a positive correlation between leaf gas film and increased root pO2. Combining laboratory and in situ measurements thus contributed to understanding how leaf gas films help the rice plant survive flooding.
Related Publications
Related Products
High performance oxygen microsensor

UniAmp Multi Channel for all Unisense sensors and electrodes including optical sensors
Microprofiles with extreme accuracy, high spatial and temporal resolution

Water resistant Field MicroProfiling System to generate, study, and analyze microprofiles

Optical oxygen sensor technology
Detect hydrogen sulfide in your sample
Measure dissolved and gaseous hydrogen with a high-precision

Optode meter for accurate quantification

Measure dissolved and gaseous nitrous oxide
Measure minute concentrations of nitric oxide
Miniaturized pH electrode
Measure redox potential in microenvironments

Monitor temperature in your sample

8-channel microsensor amplifier for in situ measurements
Sealed system to measure O2, H2, H2S, N2O, NO, pH, Redox, and temperature metabolism
