
Understanding the Denitrification Dynamics of Nitrifying Microorganisms
Introduction
Nitrifying microorganisms are ubiquitous in the environment and perform the essential step of nitrification in the biogeochemical global nitrogen cycle. Depending on the environment, the process of nitrification can be viewed as a positive and desirable process (during wastewater treatment) or as a negative and undesired process (leading to agricultural fertilizer loss).
Interestingly, under anoxic conditions, some nitrifying microorganisms are also able to perform the process of denitrification which produces nitric oxide (NO) and nitrous oxide (N2O). Therefore, regulating where nitrification is occurring, and where nitrifying microorganisms are active in the environment is an important part of our global nitrogen management strategy. To accomplish this, the first step is to characterize the nitrifying microorganisms themselves.
With our research we aim to determine how nitrifying microorganisms are able to produce nitrous oxide through denitrification. We use an approach that combines comparative genomics and culture-based physiological assays. Together, these techniques allow us to identify genes or proteins that are essential for the process, along with being able to describe different physiological phenotypes. The Unisense MicroRespiration System enables us to look at the physiological process of microbially driven denitrification under highly controlled conditions.

Laboratory setup
A MicroRespiration System equipped with a 10 mL double MR chamber and two injections lids was used to monitor the production and consumption profile of NO and N2O during anoxic denitrification. An oxygen optode was attached to the side of the chamber and NO / N2O microsensors were used in order to measure O2, NO, and N2O simultaneously (Figure 1). Here, the MR chamber was filled headspace-free with a pure nitrifying microorganism culture. After an initial substrate injection, microbial respiration depleted the dissolved oxygen in the MR chamber. Once anoxic, nitrite was injected and the production and consumption of NO and N2O was observed.
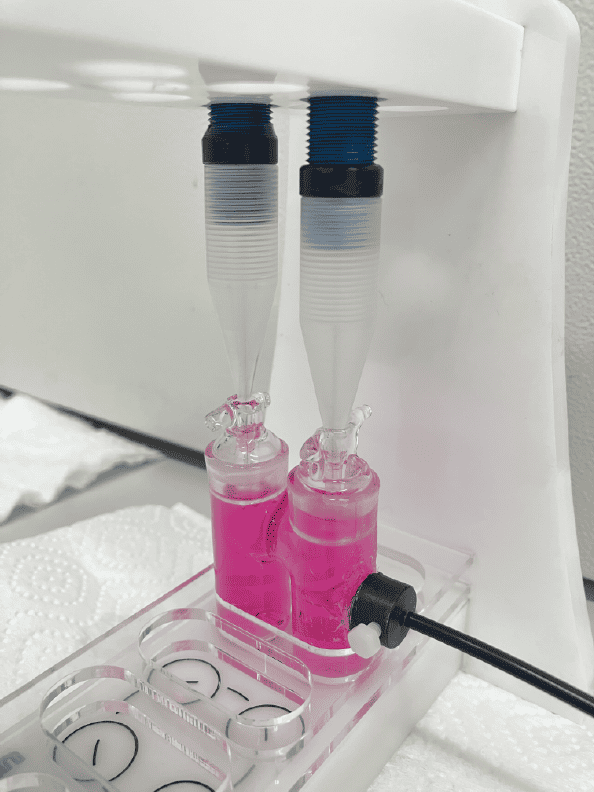
Results and Conclusion
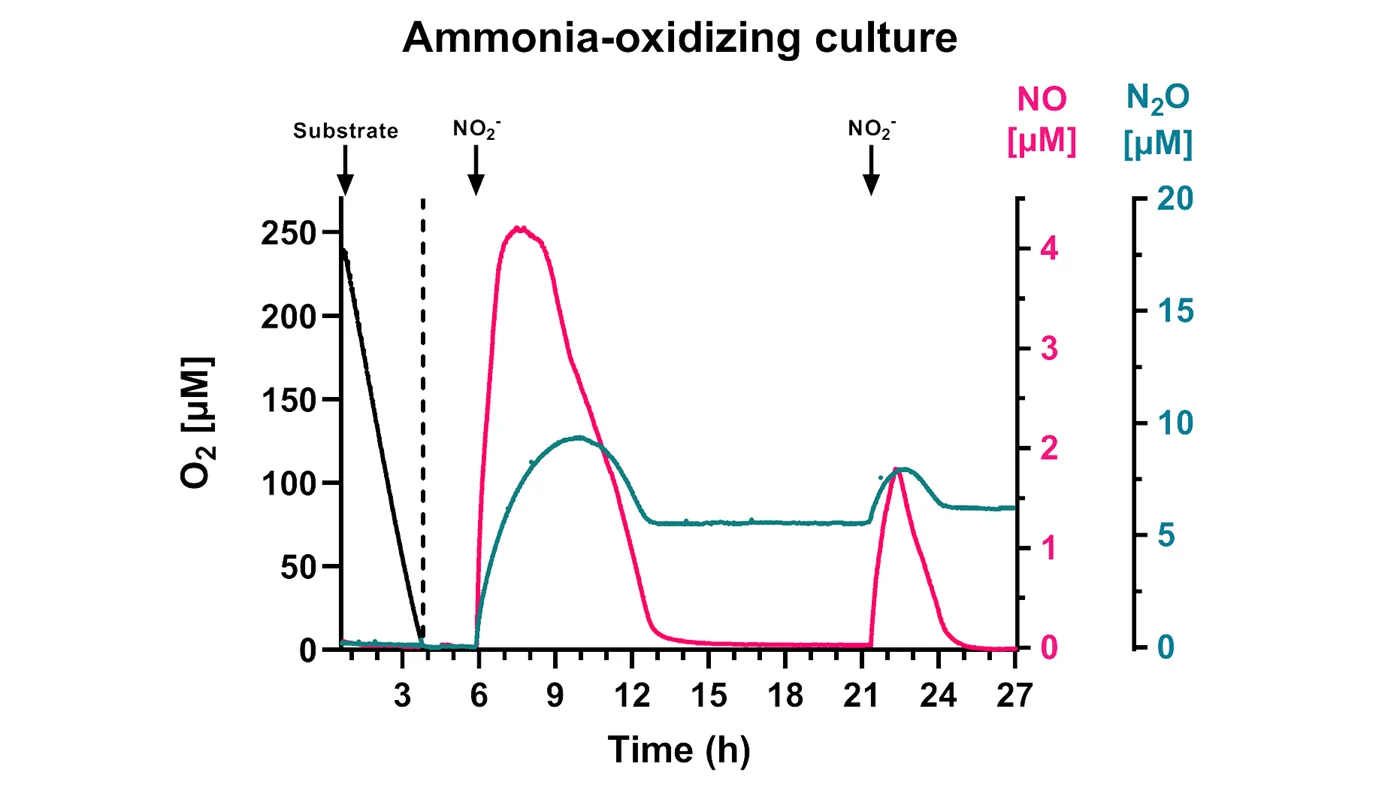
One example of an anoxic NO and N2O production and consumption profile of a nitrifying microorganism is provided (Figure 2). The introduction of nitrite into the anoxic MR chamber is immediately followed by a burst of NO and N2O production. Here, over the course of ~9 hours, the NO intermediate is completely depleted, but there is a stable amount of N2O that remains. As shown, multiple nitrite injections can be performed subsequently in a single chamber. Each time, NO is produced an intermediate which is fully consumed after several hours, while a stable amount of N2O remains.
Although only a single example is shown here, the NO and N2O production and consumption profiles of different species of nitrifying microorganisms can be compared using this method. These profiles, together with comparative genomics, can be used to identify and characterize the essential genes needed for nitrifier denitrification. With this information, the ability of individual nitrifier species or nitrifier communities to produce N2O can more accurately be predicted solely with insilico or molecular techniques.
Comment from Dr. Chris Sedlacek
“The Unisense suite of sensors provides our research team the flexibility we need to investigate the profiles of several dissolved gases simultaneously. Great products, helpful people!”.
The application note was written by
Dr. Chris Sedlacek
University of Vienna
Centre for Microbiology and Environmental Systems Science
Division of Microbial Ecology
Related publications
Related products
High performance oxygen microsensor

Optical oxygen sensor technology

Measure dissolved and gaseous nitrous oxide
Measure minute concentrations of nitric oxide

Stable and easy handling of the sensor during MicroRespiration measurements
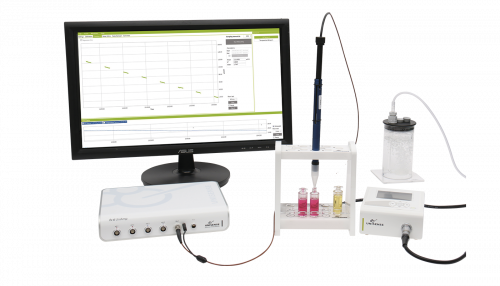
Sealed system to measure O2, H2, H2S, N2O, NO, pH, Redox, and temperature metabolism
Microrespiration experiments: measure respiration and production rates.
