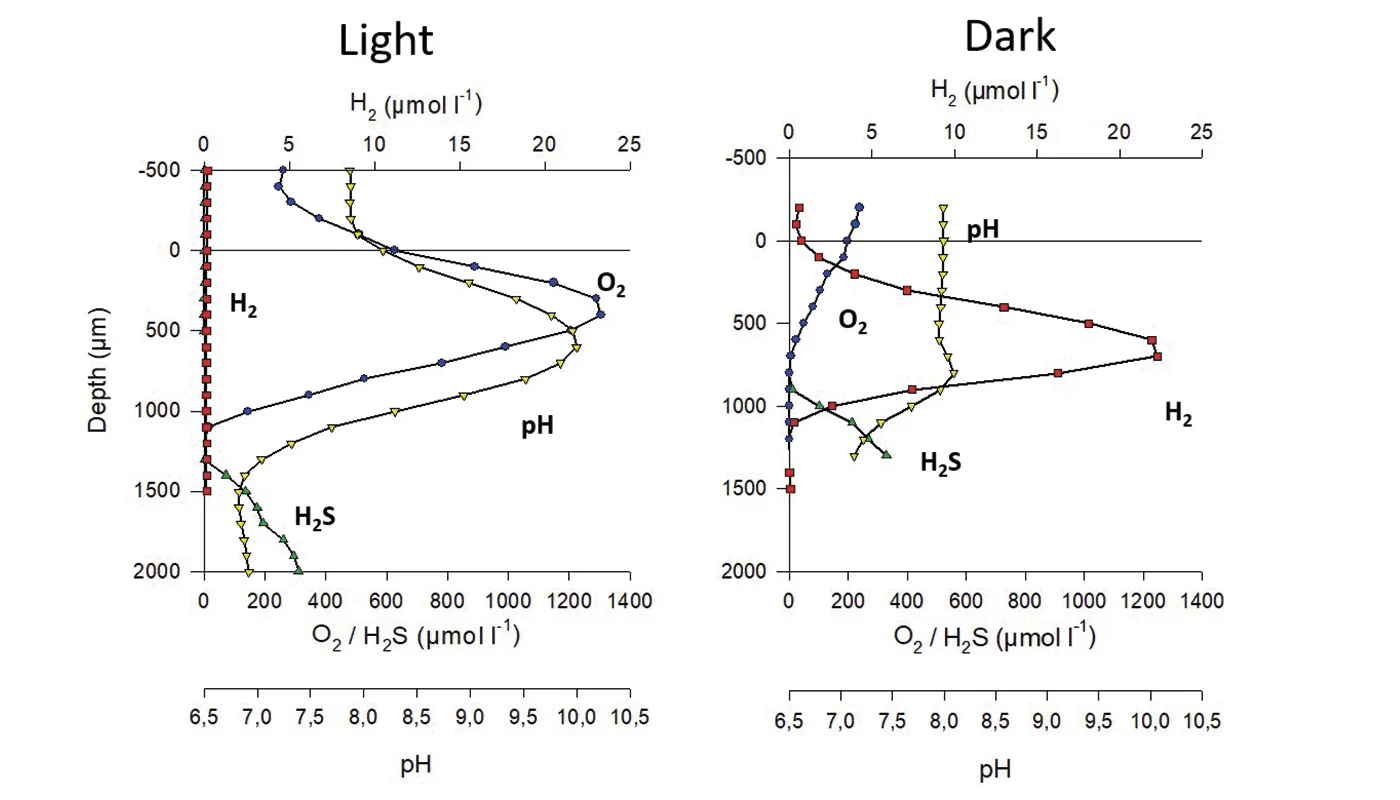
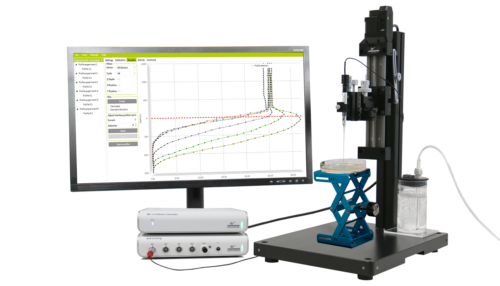
Oxygen evolution in a hypersaline crust:in situ photosynthesis quantification by microelectrode profiling…
Woelfel, Jana et al. (2009),
Aquatic Microbial Ecology,
vol. 56,
263-273
Woelfel, Jana, Sørensen, Ketil, Warkentin, Mareike, Forster, Stefan, Oren, Aharon, Schumann, Rhena (2009),
Aquatic Microbial Ecology,
vol.
56,
263-273
Net primary production and respiration were estimated in a hypersaline cyanobacterial mat colonizing a gypsum crust in the Eilat salterns, Israel. Two different approaches were used: in situ microprofiling with Clark-type O 2 sensors and application of optode sensor spots in incubation chambers. The net O2 release rates of the mat phototrophs was high, with a maximum of 3.4 nmol O2 cm-2min-1measured by microprofiling and 4.4 nmol O2 cm-2min -1determined in the incubation chambers. The upper 2 layers of the mat as well as the overlying water quickly became O2 saturated during the day. The respiration of the whole gypsum crust was also very intensive and corresponded to the O2 produced by photosynthesis on a diurnal basis, which prevented most of the evolved O2 from reaching the water. The results presented show that optode sensor spots are useful tools providing additional information about export and photosynthetic production rates of O2 in hypersaline microbial mats. © Inter-Research 2009.
10.3354/ame01326
Biofilm Growth and Near-Infrared Radiation-Driven Photosynthesis of the Chlorophyll d-Containing Cyanobac…
Behrendt, Lars et al. (2012),
Applied and Environmental Microbiology,
vol. 78,
3896-3904
Behrendt, Lars, Schrameyer, Verena, Qvortrup, Klaus, Lundin, Luisa, Sørensen, Søren J., Larkum, Anthony W.D., Kühl, Michael (2012),
Applied and Environmental Microbiology,
vol.
78,
3896-3904
The cyanobacterium Acaryochloris marina is the only known phototroph harboring chlorophyll (Chl) d. It is easy to cultivate it in a planktonic growth mode, and A. marina cultures have been subject to detailed biochemical and biophysical characterization. In natural situations, A. marina is mainly found associated with surfaces, but this growth mode has not been studied yet. Here, we show that the A. marina type strain MBIC11017 inoculated into alginate beads forms dense biofilm-like cell clusters, as in natural A. marina biofilms, characterized by strong O2 concentration gradients that change with irradiance. Biofilm growth under both visible radiation (VIS, 400 to 700 nm) and near-infrared radiation (NIR, ~ 700 to 730 nm) yielded maximal cell-specific growth rates of 0.38 per day and 0.64 per day, respectively. The population doubling times were 1.09 and 1.82 days for NIR and visible light, respectively. The photosynthesis versus irradiance curves showed saturation at a photon irradiance of Ek (saturating irradiance) >250 μmol photonsm-2 s-1 for blue light but no clear saturation at 365 μmol photonsm -2 s-1 for NIR. The maximal gross photosynthesis rates in the aggregates were ~ 1,272 μmol O2 mg Chl d -1 h -1 (NIR) and ~ 1,128 μmol O2 mg Chl d-1 h-1 (VIS). The photosynthetic efficiency (α) values were higher in NIR-irradiated cells [(268 ± 0.29) × 10-6m2 mg Chl d-1 (mean ± standard deviation)] than under blue light [(231 ± 0.22) × 10-6m2 mg Chl d-1]. A. marina is well adapted to a biofilm growth mode under both visible and NIR irradiance and under O2 conditions ranging from anoxia to hyperoxia, explaining its presence in natural niches with similar environmental conditions. © 2012, American Society for Microbiology.
10.1128/AEM.00397-12
Microenvironments and microbial community structure in sediments
Tankéré, S. P.C. et al. (2002),
Environmental Microbiology,
vol. 4,
97-105
Tankéré, S. P.C., Bourne, D. G., Muller, F. L.L., Torsvik, V. (2002),
Environmental Microbiology,
vol.
4,
97-105
The aim of this study was to explore the potential of a combined chemical and microbiological approach as part of a study of organic carbon oxidation processes in sediments. An assessment of microbiological diversity using molecular techniques was carried out in combination with high resolution chemical measurements at the sediment-water interface of a coastal lagoon affected by eutrophication in autumn 2000. There was a 0.2 mm overlap between the O2 and H2S profiles. pH showed a maximum just above the sediment-water interface coinciding with an oxygen maximum, suggesting photosynthetic activity, and a minimum coinciding with the O22-H2S interface. The redox potential was high in bottom water and surface sediment, reflecting the presence of oxygen and oxides, and reached low values after a step-wise decrease at -18 mm. Reduction of Fe occurred within the biofilm at the O2-H2S interface and was mostly due to reduction by H2S. The elevated concentrations of dissolved Mn in the oxic water may have been caused either by in situ production within organic aggregates or lateral water flow from sites nearby at which Mn2+ diffuses out of the sediment. Sequences related to sulphur chemolitotrophs were retrieved from the biofilm samples, which is consistent with the small overlap between O2 and H2S observed in this biofilm. Although the resolution of techniques used was different, sequencing results were consistent with chemical data in delineating the same horizons according to redox, pH or ecological properties.
10.1046/j.1462-2920.2002.00274.x
Microbial diversity of biofilm communities in microniches associated with the didemnid ascidian Lissoclinum patella
Behrendt, Lars et al. (2012),
ISME Journal,
vol. 6,
1222-1237
Behrendt, Lars, Larkum, Anthony W.D., Trampe, Erik, Norman, Anders, Sørensen, Søren J., Kühl, Michael (2012),
ISME Journal,
vol.
6,
1222-1237
We assessed the microbial diversity and microenvironmental niche characteristics in the didemnid ascidian Lissoclinum patella using 16S rRNA gene sequencing, microsensor and imaging techniques. L. patella harbors three distinct microbial communities spatially separated by few millimeters of tunic tissue: (i) a biofilm on its upper surface exposed to high irradiance and O 2 levels, (ii) a cloacal cavity dominated by the prochlorophyte Prochloron spp. characterized by strong depletion of visible light and a dynamic chemical microenvironment ranging from hyperoxia in light to anoxia in darkness and (iii) a biofilm covering the underside of the animal, where light is depleted of visible wavelengths and enriched in near-infrared radiation (NIR). Variable chlorophyll fluorescence imaging demonstrated photosynthetic activity, and hyperspectral imaging revealed a diversity of photopigments in all microhabitats. Amplicon sequencing revealed the dominance of cyanobacteria in all three layers. Sequences representing the chlorophyll d containing cyanobacterium Acaryochloris marina and anoxygenic phototrophs were abundant on the underside of the ascidian in shallow waters but declined in deeper waters. This depth dependency was supported by a negative correlation between A. marina abundance and collection depth, explained by the increased attenuation of NIR as a function of water depth. The combination of microenvironmental analysis and fine-scale sampling techniques used in this investigation gives valuable first insights into the distribution, abundance and diversity of bacterial communities associated with tropical ascidians. In particular, we show that microenvironments and microbial diversity can vary significantly over scales of a few millimeters in such habitats; which is information easily lost by bulk sampling. © 2012 International Society for Microbial Ecology All rights reserved.
10.1038/ismej.2011.181
Influence of uranium (VI) on the metabolic activity of stable multispecies biofilms studied by oxygen mic…
Krawczyk-Bärsch, Evelyn et al. (2008),
Geochimica et Cosmochimica Acta,
vol. 72,
5251-5265
Krawczyk-Bärsch, Evelyn, Grossmann, Kay, Arnold, Thuro, Hofmann, Susann, Wobus, Axel (2008),
Geochimica et Cosmochimica Acta,
vol.
72,
5251-5265
The effect of uranium added in ecologically relevant concentrations (1 × 10-5 and 1 × 10-6 M) to stable multispecies biofilms was studied by electrochemical oxygen microsensors with tip diameters of 10 μm and by confocal laser fluorescence microscopy (CLSM). The microsensor profile measurements in the stable multispecies biofilms exposed to uranium showed that the oxygen concentration decreased faster with increasing biofilm depth compared to the uranium free biofilms. In the uranium containing biofilms, the oxygen consumption, calculated from the steady-state microprofiles, showed high consumption rates of up to 61.7 nmol cm-3 s-1 in the top layer (0-70 μm) and much lower consumption rates in the lower zone of the biofilms. Staining experiments with 5-cyano-2,3-ditolyl tetrazolium chloride (CTC) and 4,6-diamidino-2-phenylindole (DAPI) confirmed the high respiratory activities of the bacteria in the upper layer. Analysis of the amplified 16S rRNA gene fragments showed that the addition of uranium in ecologically relevant concentrations did not change the bacterial diversity in the stable multispecies biofilms and is therefore not responsible for the different oxygen profiles in the biofilms. The fast decrease in the oxygen concentrations in the biofilm profiles showed that the bacteria in the top region of the biofilms, i.e., the metabolically most active biofilm zone, battle the toxic effects of aqueous uranium with an increased respiratory activity. This increased respiratory activity results in O2 depleted zones closer to the biofilm/air interface which may trigger uranium redox processes, since suitable redox partners, e.g., extracellular polymeric substance (EPS) and other organics (e.g., metabolites), are sufficiently available in the biofilm porewaters. Such redox reactions may lead to precipitation of uranium (IV) solids and consequently to a removal of uranium from the aqueous phase. © 2008 Elsevier Ltd. All rights reserved.
10.1016/j.gca.2008.07.029