Fast escape of hydrogen from gas cavities around corroding magnesium implants
Kuhlmann, Julia et al. (2013),
Acta Biomaterialia,
vol. 9,
8714-8721
Kuhlmann, Julia, Bartsch, Ivonne, Willbold, Elmar, Schuchardt, Sven, Holz, Olaf, Hort, Norbert, Höche, Daniel, Heineman, William R, Witte, Frank, Heineman, William R. (2013),
Acta Biomaterialia,
vol.
9,
8714-8721
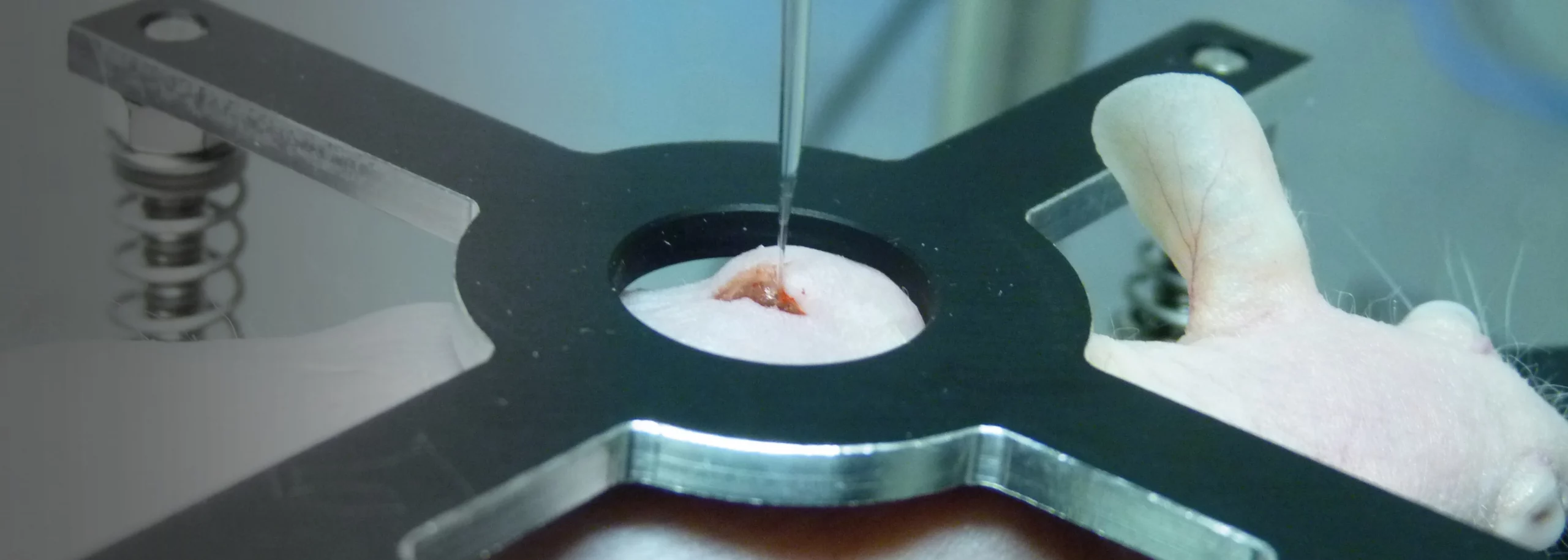
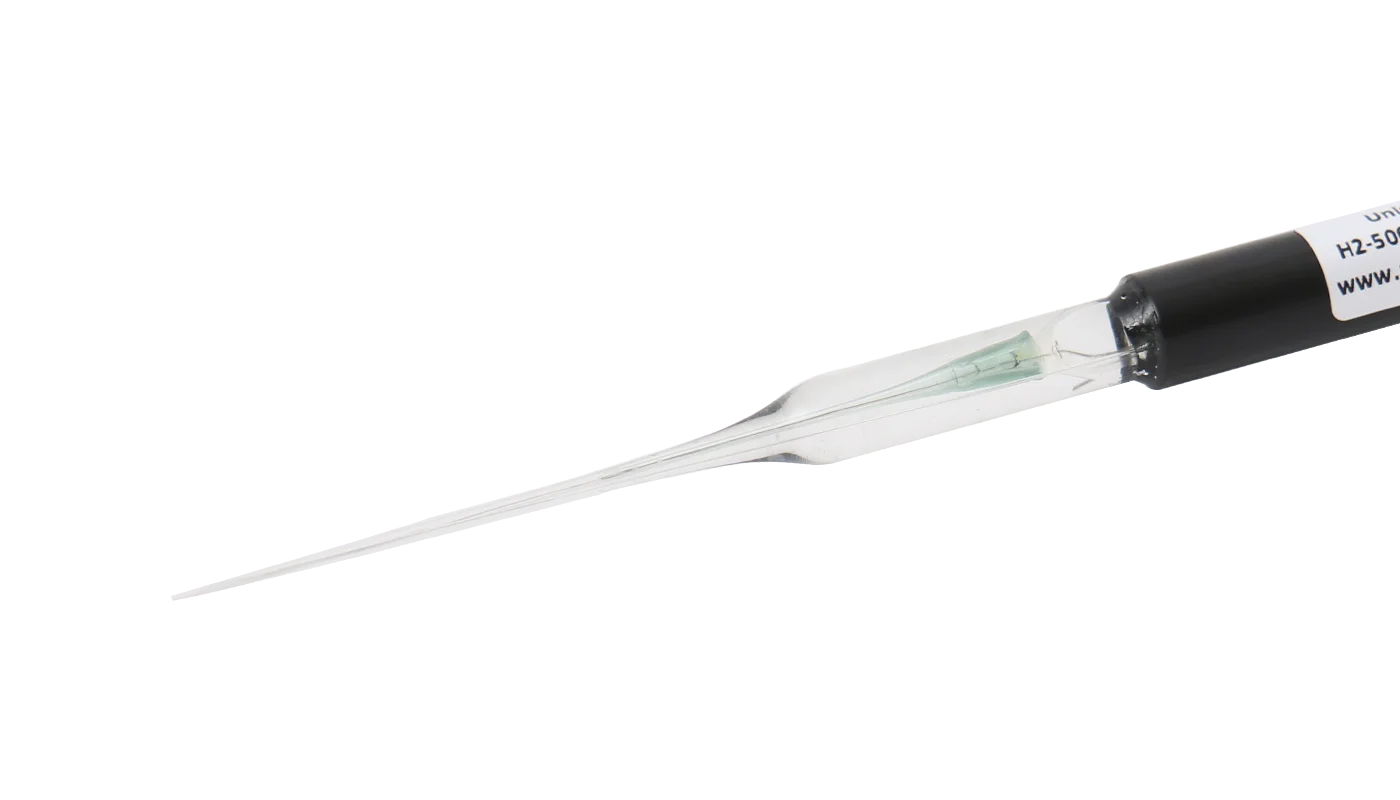
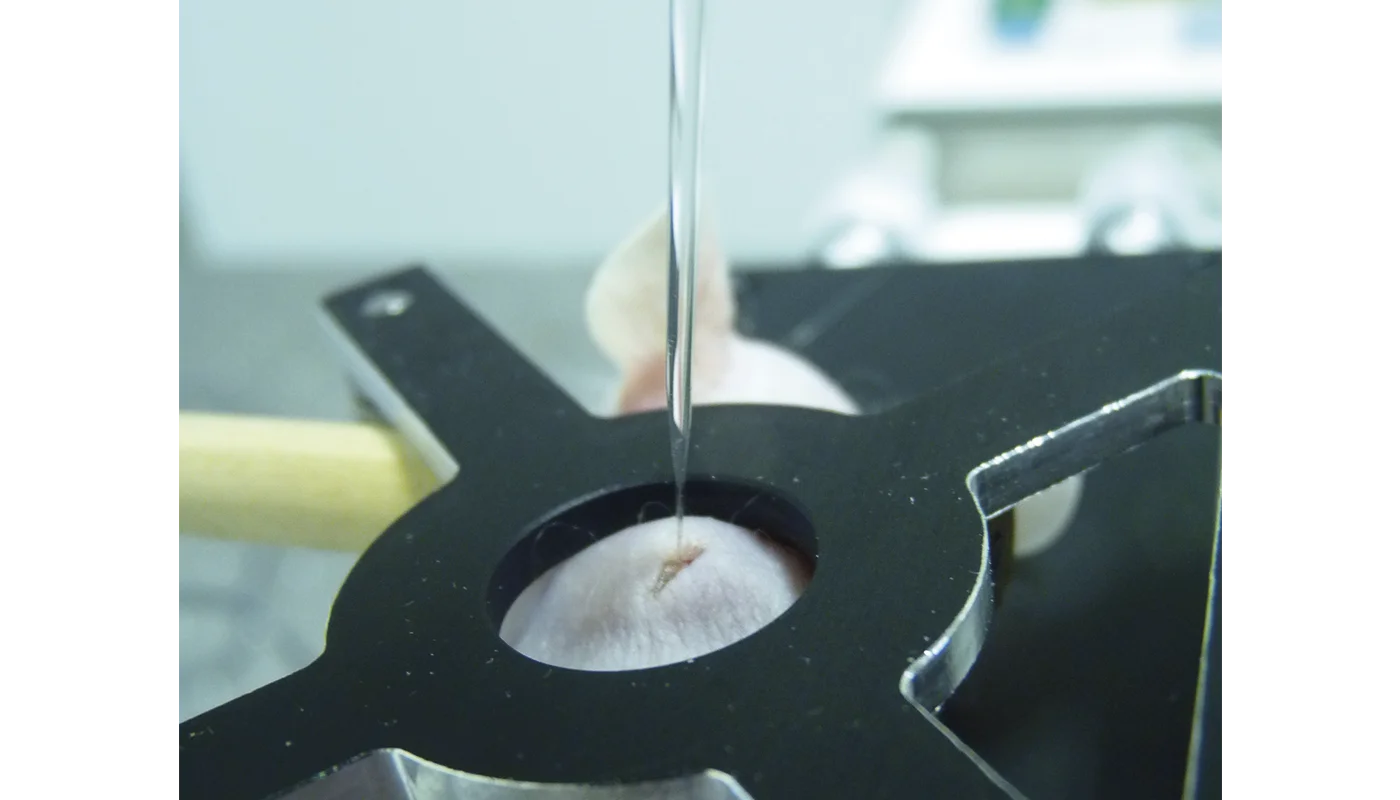
Magnesium materials are of increasing interest in the development of biodegradable implants as they exhibit properties that make them promising candidates. However, the formation of gas cavities after implantation of magnesium alloys has been widely reported in the literature. The composition of the gas and the concentration of its components in these cavities are not known as only a few studies using non-specific techniques were done about 60 years ago. Currently many researchers assume that these cavities contain primarily hydrogen because it is a product of magnesium corrosion in aqueous media. In order to clearly answer this question we implanted rare earth-containing magnesium alloy disks in mice and determined the concentration of hydrogen gas for up to 10 days using an amperometric hydrogen sensor and mass spectrometric measurements. We were able to directly monitor the hydrogen concentration over a period of 10 days and show that the gas cavities contained only a low concentration of hydrogen gas, even shortly after formation of the cavities. This means that hydrogen must be exchanged very quickly after implantation. To confirm these results hydrogen gas was directly injected subcutaneously. Most of the hydrogen gas was found to exchange within 1 h after injection. Overall, our results disprove the common misbelief that these cavities mainly contain hydrogen and show how quickly this gas is exchanged with the surrounding tissue. © 2012 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
10.1016/j.actbio.2012.10.008
In vivo quantification of hydrogen gas concentration in bone marrow surrounding magnesium fracture fixati…
Zhao, Daoli et al. (2018),
Acta Biomaterialia,
vol. 73,
559-566
Zhao, Daoli, Brown, Andrew, Wang, Tingting, Yoshizawa, Sayuri, Sfeir, Charles, Heineman, William R. (2018),
Acta Biomaterialia,
vol.
73,
559-566
Magnesium (Mg) medical devices are currently being marketed for orthopedic applications and have a complex degradation process which includes the evolution of hydrogen gas (H2). The effect of H2 exposure on relevant cell types has not been studied; and the concentration surrounding degrading Mg devices has not been quantified to enable such mechanistic studies. A simple and effective method to measure the concentration of H2 in varying microenvironments surrounding Mg implants is the first step to understanding the biological impact of H2 on these cells. Here, the in vivo measurement of H2 surrounding fracture fixation devices implanted in vivo is demonstrated. An electrochemical H2 microsensor detected increased levels of H2 at three anatomical sites with a response time of about 30 s. The sensor showed the H2 concentration in the bone marrow at 1 week post-implantation (1460 ± 320 µM) to be much higher than measured in the subcutaneous tissue (550 ± 210 µM) and at the skin surface (120 ± 50 µM). Additionally, the H2 concentrations measured in the bone marrow exceeded the concentration in a H2 saturated water solution (∼800 µM). These results suggest that H2 emanating from Mg implants in bone during degradation pass through the bone marrow and become at least partially trapped because of slow permeation through the bone. This study is the first to identify H2 concentrations in the bone marrow environment and will enable in vitro experiments to be executed at clinically relevant H2 concentrations to explore possible biological effects of H2 exposure. Statement of Significance: An electrochemical H2 sensor was used to monitor the degradation of a Mg fracture fixation system in a lapine ulna fracture model. Interestingly, the H2 concentration in the bone marrow is 82% higher than H2 saturated water solution. This suggests H2 generated in situ is trapped in the bone marrow and bone is less permeable than the surrounding tissues. The detectable H2 at the rabbit skin also demonstrates a H2 sensor's ability to monitor the degradation process under thin layers of tissue. H2 sensing shows promise as a tool for monitoring the degradation of Mg alloy in vivo and creating in vitro test beds to more mechanistically evaluate the effects of varying H2 concentrations on cell types relevant to osteogenesis.
10.1016/j.actbio.2018.04.032
Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabol…
Kamimura, Naomi et al. (2011),
Obesity,
vol. 19,
1396-1403
Kamimura, Naomi, Nishimaki, Kiyomi, Ohsawa, Ikuroh, Ohta, Shigeo (2011),
Obesity,
vol.
19,
1396-1403
Recent extensive studies have revealed that molecular hydrogen (H 2) has great potential for improving oxidative stress-related diseases by inhaling H2 gas, injecting saline with dissolved H 2, or drinking water with dissolved H2 (H 2-water); however, little is known about the dynamic movement of H2 in a body. First, we show that hepatic glycogen accumulates H 2 after oral administration of H2-water, explaining why consumption of even a small amount of H2 over a short span time efficiently improves various disease models. This finding was supported by an in vitro experiment in which glycogen solution maintained H2. Next, we examined the benefit of ad libitum drinking H2-water to type 2 diabetes using db/db obesity model mice lacking the functional leptin receptor. Drinking H2-water reduced hepatic oxidative stress, and significantly alleviated fatty liver in db/db mice as well as high fat-diet-induced fatty liver in wild-type mice. Long-term drinking H2-water significantly controlled fat and body weights, despite no increase in consumption of diet and water. Moreover, drinking H2-water decreased levels of plasma glucose, insulin, and triglyceride, the effect of which on hyperglycemia was similar to diet restriction. To examine how drinking H2-water improves obesity and metabolic parameters at the molecular level, we examined gene-expression profiles, and found enhanced expression of a hepatic hormone, fibroblast growth factor 21 (FGF21), which functions to enhance fatty acid and glucose expenditure. Indeed, H2 stimulated energy metabolism as measured by oxygen consumption. The present results suggest the potential benefit of H2 in improving obesity, diabetes, and metabolic syndrome. © 2011 The Obesity Society.
10.1038/oby.2011.6
The protective role of hydrogen-rich saline in experimental liver injury in mice
Han, Yong, Sun et al. (2011),
Journal of Hepatology,
vol. 54,
471-480
Han, Yong, Sun, L., Chen, W., Zhou, L., Hu, L., Li, Q., Tu, Y., Chang, Q., Liu, X., Sun, M., Wu, Sun, Hanyong, Chen, Lei, Zhou, Weiping, Hu, Liang, Li, Liang, Tu, Qianqian, Chang, Yanxin, Liu, Qu, Sun, Xuejun, Wu, Mengchao, Wang, Hongyang (2011),
Journal of Hepatology,
vol.
54,
471-480
Background & Aims: Reactive oxygen species (ROS) are considered to play a prominent causative role in the development of various hepatic disorders. Antioxidants have been effectively demonstrated to protect against hepatic damage. Hydrogen (H 2), a new antioxidant, was reported to selectively reduce the strongest oxidants, such as hydroxyl radicals (OH) and peroxynitrite (ONOO -), without disturbing metabolic oxidation-reduction reactions or disrupting ROS involved in cell signaling. In place of H 2 gas, hydrogen-rich saline (HS) may be more suitable for clinical application. We herein aim to verify its protective effects in experimental models of liver injury. Methods: H 2 concentration in vivo was detected by hydrogen microelectrode for the first time. Liver damage, ROS accumulation, cytokine levels, and apoptotic protein expression were, respectively, evaluated after GalN/LPS, CCl 4, and DEN challenge. Simultaneously, CCl 4-induced hepatic cirrhosis and DEN-induced hepatocyte proliferation were measured. Results: HS significantly increased hydrogen concentration in liver and kidney tissues. As a result, acute liver injury, hepatic cirrhosis, and hepatocyte proliferation were reduced through the quenching of detrimental ROS. Activity of pro-apoptotic players, such as JNK and caspase-3, were also inhibited. Conclusions: HS could protect against liver injury and also inhibit the processes leading to liver cirrhosis and hepatocyte compensatory proliferation. © 2010 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
10.1016/j.jhep.2010.08.011