
The Winning Formula
Professor Ole Pedersen at University of Copenhagen is a very experienced microsensor user with a long track record of published articles where Unisense microsensors have been a key technology. Ole has travelled the world with microsensors and applied them in seagrass beds in the Caribbean Sea, rice paddies in the Philippines and peat swamps in Australia just to mention a few exotic research locations. A number of Ole’s publications are based on initiating microsensor studies in the laboratory and subsequently taking the measurements to the field, a strategy that he calls “the winning formula”. Unisense invited Ole for a talk about microsensor research and the so-called winning formula.
Ole’s important take-home messages:
- Include in situ measurements - it is a winning formula
- Learn your system in the laboratory before going in situ
- Choose the right size of sensor to answer your question
- Avoid breaking sensors in a wobbly experimental setup

What are the typical challenges you meet when setting up a microsensor experiment?
It’s all about stability and ease of access! Stability – no unsteady tables or shaky tanks and containers – is the main thing in any microsensor set-up. I use a lot of time to ensure that the pots, the cores, or the trays where I have my specimens are well fixed, e.g. to the table or the bottom of the tank. Then, I fix all cables, and also the leaves if working with plants, to avoid that I break a sensor already in the process of mounting it in the micromanipulator… My laboratory set-ups look very “clean”.

What were your initial reasons for going into the field?
Curiosity! I started with laboratory measurements in seagrasses and got fantastic data. But the uncertainty of whether the observed phenomena also occurred in the field situation was there
– so I had to go and see.
What would you state as the greatest advantages of starting the experiment in the laboratory and then proceed into the field?
You know exactly what you are looking for! I strongly prefer to identify a mechanism in the lab where I can control all the environmental parameters such as temperature, light, flow – and
stability of the set-up. The interesting part for me and the readers of the scientific papers is then to go and show that this mechanism also operate in the field situation regardless of fluctuations
in all of the above environmental parameters.

Then, what is the greatest challenge you have experienced working in the field?
Working in a peat swamp in South West Australia was probably the greatest challenge! The water was shallow so I didn’t have to SCUBA dive but the peat swamp was incredibly unstable. Walking 5 m away from the set-up would cause the entire set-up with micromanipulators and sensors to move. I had to float around and never touch the bottom in order to get the sensors in place.

Could you give examples of when combining field and laboratory measurements were of a particular advantage?
Working with submerged rice – or seagrasses – in the field situation has provided new insight into internal aeration of plant tissues. Our previous laboratory measurements have not been
able to provide real knowledge of the importance of simultaneous changes in light, temperature and water flow and how these parameters all affected the oxygen status of the tissues.
The exciting data on the following pages of this flyer speaks for itself and I hope it will stimulate other research groups to try and combine laboratory and field measurements regardless of the
topic they are working on.

Why does in situ data impress reviewers? Why is it so persuading?
Field measurements are impressive to reviewers and readers because they already know how hard it is to do it in the controlled laboratory situation. Even in the laboratory it is difficult enough
to position a microsensor in the tissue right where it is needed. Research in the field is challenging but the reward is immense!
A standard question we get from new customers is how often sensors break during experiments. What is your experience?
I rarely break a sensor during measurements! Sensors break during handling i.e. when removing it from its protective casing, mounting it in the micromanipulator or inserting it into the tissue. Once the set-up is up and running, the microsensors rarely break – unless the set-up is unstable or macrofauna is attracted to the set-up and starts fiddling with it. The bull sharks in Florida Bay completely wrecked a set-up.
Any good advice to new microsensor users? Patience! It takes a while before you have achieved the necessary skills. But once you feel comfortable with the data you get, it is so rewarding to get insight into processes and mechanisms that
nobody has studied before.

A standard question we get from new customers is how often sensors break during experiments. What is your experience?
I rarely break a sensor during measurements! Sensors break during handling i.e. when removing it from its protective casing, mounting it in the micromanipulator or inserting it into the tissue. Once the set-up is up and running, the microsensors rarely break – unless the set-up is unstable or macrofauna is attracted to the set-up and starts fiddling with it. The bull sharks in Florida Bay completely wrecked a set-up.
Any good advice to new microsensor users? Patience! It takes a while before you have achieved the necessary skills. But once you feel comfortable with the data you get, it is so rewarding to get insight into processes and mechanisms that
nobody has studied before.

Ready to dive into your own measurements?
Contact us today to discuss whether microsensors are the right choice for your research application.
Related Products
High performance oxygen microsensor

UniAmp Multi Channel for all Unisense sensors and electrodes including optical sensors
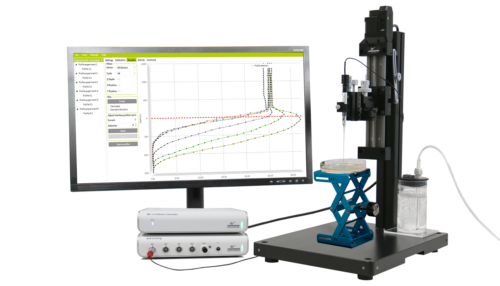
Microprofiles with extreme accuracy, high spatial and temporal resolution

Water resistant Field MicroProfiling System to generate, study, and analyze microprofiles

Optical oxygen sensor technology
Detect hydrogen sulfide in your sample

Economic amplifier portfolio for single analytes - O2, pH/mV, H2, N2O or H2S
Measure dissolved and gaseous hydrogen with a high-precision

Optode meter for accurate quantification

Measure dissolved and gaseous nitrous oxide
Measure minute concentrations of nitric oxide
Miniaturized pH electrode
Measure redox potential in microenvironments
Microelectrode to measure electric potential

Reference electrode for pH or Redox microelectrodes
