
Inhibition of Metabolic Activity in Acute Myeloid Leukemia
Introduction
Acute myeloid leukemia (AML) is a fast-growing cancer characterized by poorly differentiated, abnormal white blood cells. These abnormal blood cells crowd healthy blood cells causing fatigue, increased bleeding tendency, and reduced immune defense. AML is the most common type of leukemia, and it is notoriously difficult to treat due to resistance, heterogeneity and rapid progress.
“The Unisense system has been beneficial to our research by enabling measurements in demanding experimental setups and supporting the generation of high-quality data. We have also had positive experiences with their technical support, which has been both responsive and knowledgeable.”
Associate Prof. Lotte Bonde Bertelsen, MR Research Centre, Aarhus University
Abnormal blood cells in AML have altered metabolism due to the Warburg Effect, leading to elevated glucose uptake through glycolysis - even in the presence of oxygen. This metabolic phenotype makes AML a promising candidate for treatment strategies targeting glycolytic metabolic. Among the compounds investigated, 2-deoxy-D-glucose (2-DG) has attracted significant interest. 2-DG is a glucose analog taken up by glucose transporters, which are often overexpressed in cancer. Once inside the cell, 2-DG is phosphorylated by hexokinase into a metabolite that cannot be further processed, resulting in inhibited glycolytic flux, reduced ATP production, and eventually cell death.
Christensen et al. investigated 2-DG-induced metabolic shifts in cancer cells as a proof-of-concept for targeting glycolysis in leukemia. They employed biochemical assays, hyperpolarized nuclear magnetic resonance spectroscopy, and real-time measurements of cellular respiration.
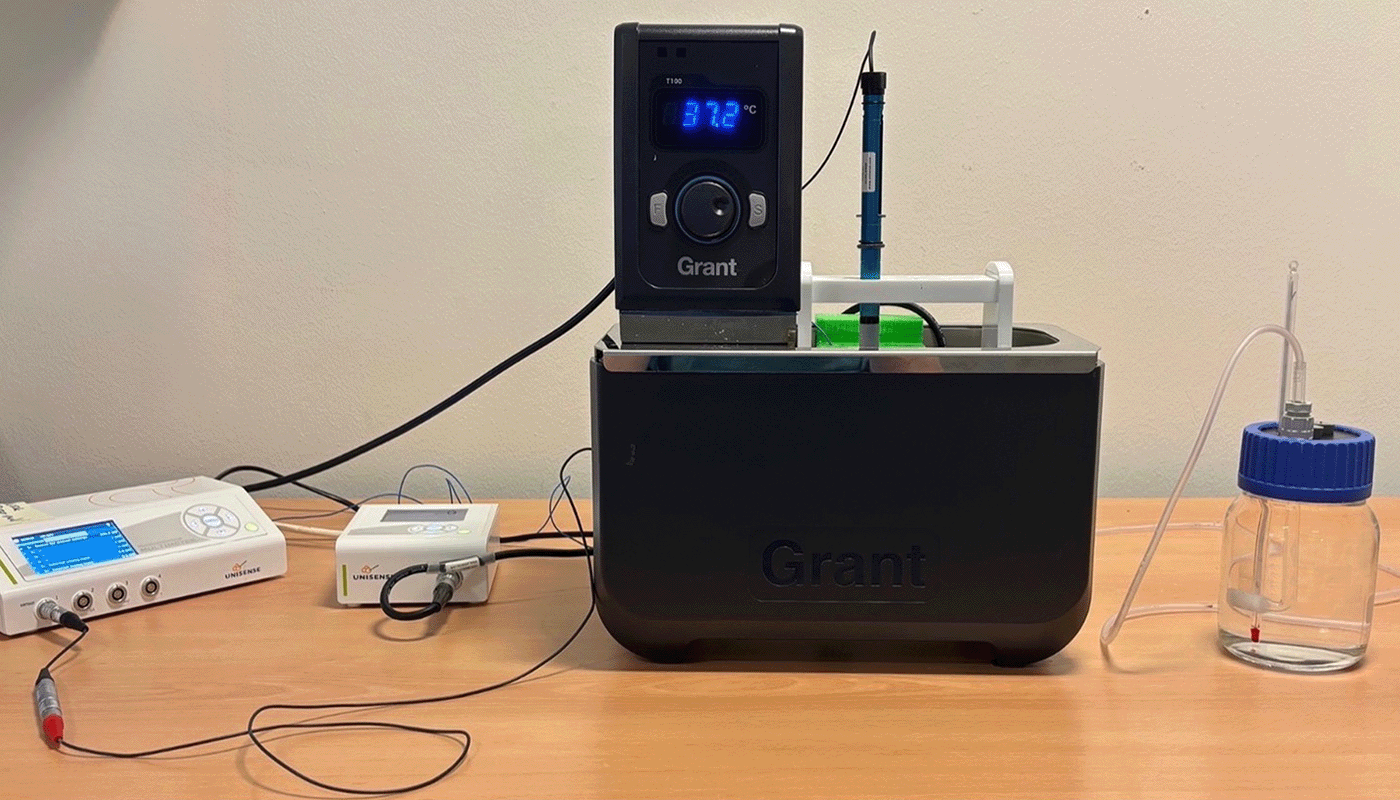
Laboratory Setup
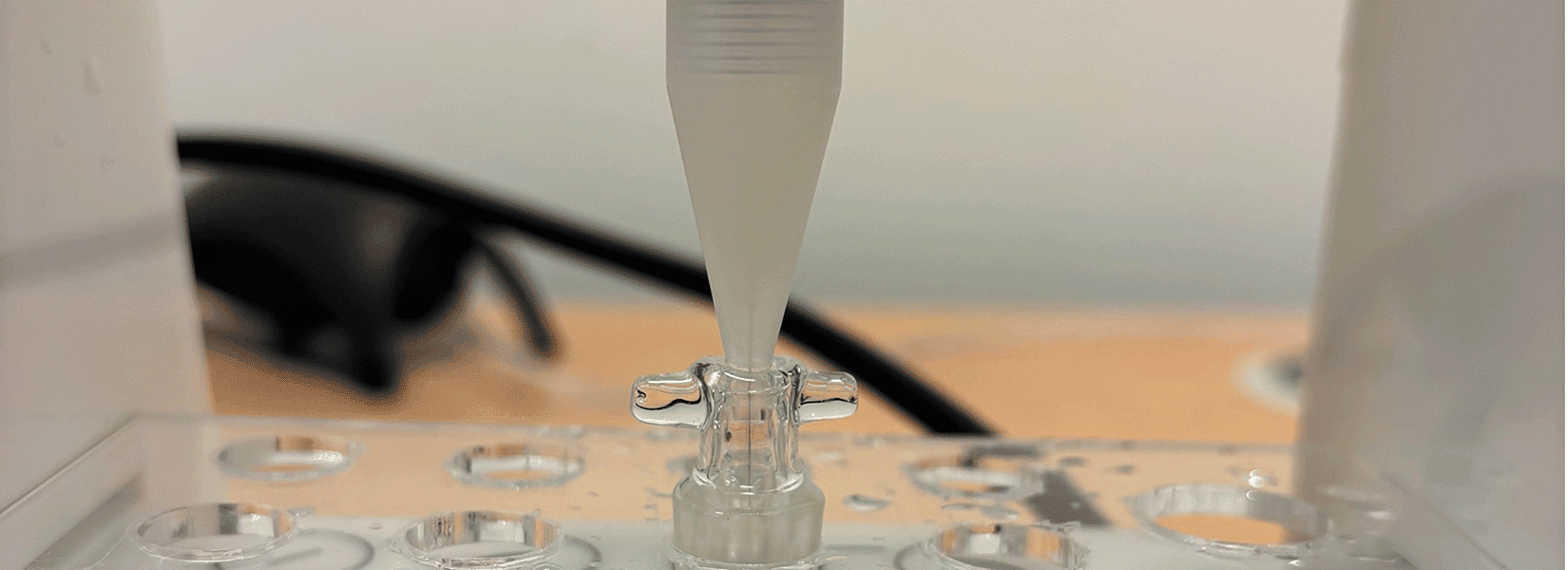
Christensen et al. assessed the effect of 2-DG on basal glycolysis, glycolytic capacity, and oxygen consumption rate using the Unisense MicroRespiration System. Cells were pre-treated with either 0, 2 or 5 mM 2-DG and subsequently resuspended in glucose and sodium pyruvate-free media. Cells were then transferred to closed Unisense MicroRespiration chambers and allowed to stabilize for 15 minutes at 37 °C before initiation of oxygen measurements (Figure 1).
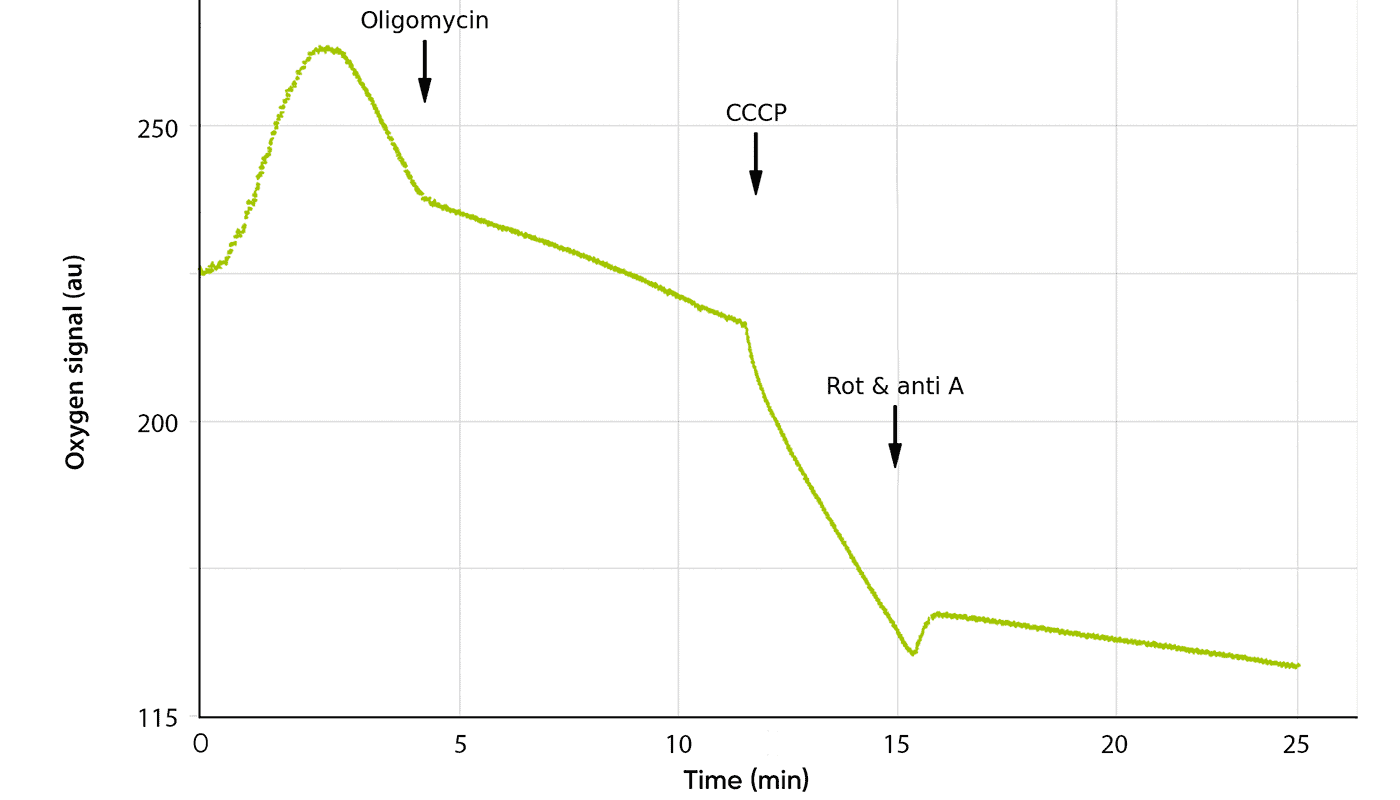
The cells were sequentially exposed to 50 mM glucose, 5 μM oligomycin, 100 mM 2-DG, and 4 μM antimycin A with 20-minute intervals between each addition. Glucose was expected to stimulate oxygen consumption, whereas oligomycin, 2-DG, and antimycin were anticipated to inhibit it. This setup enabled the researchers to monitor real-time metabolic activity during the sequential addition of metabolic inhibitors and stimulants (Figure 2).
Results and Conclusion
The oxygen consumption rate was significantly reduced in cells pre-treated with 2-DG compared to untreated controls. Furthermore, glucose only stimulated oxygen consumption in untreated cells. No stimulation was observed in cells pre-treated with 2 or 5 mM 2-DG, likely due to competitive inhibition.
Subsequent addition of 2-DG further decreased oxygen consumption – most notably in cells already exposed to 2-DG. Basal glycolysis and the glycolytic capacity, measured after oligomycin-induced inhibition of oxidative phosphorylation, were also significantly diminished.
In summary, 2-DG profoundly inhibited metabolic activity and cell proliferation, underscoring its potential to enhance chemosensitivity in AML treatment.
You can read more in the article by Christensen et al. “The effect of 2-Deoxy-D-glucose on glycolytic metabolism in acute myeloblastic leukemic ML-1 cells”, Scientific Reports, 2025 (15:17685).
Related Publications
Suggested Products
High performance oxygen microsensor

UniAmp Multi Channel for all Unisense sensors and electrodes including optical sensors

Optical oxygen sensor technology

Optode meter for accurate quantification
Sealed system to measure O2, H2, H2S, N2O, NO, pH, Redox, and temperature metabolism
Microrespiration experiments: measure respiration and production rates.
Application Notes

Bang et al. developed a dual flow chamber that mimics the shear stress and anaerobic conditions of the colon

Quantifying the Hydrogen Evolution Reaction using H2 Microsensors

Real-time measurements of oxygen consumption rates in response to 2-deoxy-D-glucose treatment

pH Microelectrode reveals distinct environment inside 3D organoid
Tissue oxygen partial pressure for screening and controlling for variation in ex vivo acute brain slice viability

How nitrifying microorganisms are able to produce nitrous oxide through denitrification

Hydrogen and oxygen microsensors for direct and real-time detection of dissolved gas in photocatalytic/electrochemical studies

The Eddy Covariance system and how to get high quality data from long term deployments with multiple sensors.

Learn in the laboratory - explore and confirm in the field!

Leaf gas films are hypothesized to improve internal aeration of the plant during the day

How to quantify the consumption rate of oxygen as well as the oxygen exchange rate across the water - sediment interface

Mitigation of N2O Emissions from Wastewater Biofilms
The use of oxygen microelectrodes to study nitritation biofilms with different geometries

O2 and N2O microprofiles in sputum samples from cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection
Oxygen and Redox Potential in Pseudomonas Aeruginosa Colony Biofilms
