
Profiling pH in Gastrointestinal Human Organoids
Introduction
Stomach ulcers are a widespread condition often triggered by infection with Helicobacter pylori, a pathogen capable of penetrating the gastric mucus layer. While this mucus is known to serve as a first line of defense, our understanding of its regulation and its coupling with other gastroprotective mechanisms (such as acid secretion) remains limited. Traditional research has relied heavily on animal models, immortalized cell lines, or mucin protein solutions, which fall short in replicating human physiology.
Human organoids - three-dimensional, multicellular structures derived from patient tissue - have emerged as a promising alternative, and they offer more physiologically relevant features. However, it remains a challenge to replicate the stomach’s extreme pH environment - particularly the steep gradient from the nearneutral epithelium to the highly acidic gastric lumen (pH 1-3).
Lyon and colleagues addressed this by leveraging tissue-derived organoid models to explore their suitability for modeling the dynamic gastric luminal environment. Conventional pH measurement methods, such as pH-sensitive dyes, suffer from limited spatial resolution and low accuracy. To overcome these limitations, the researchers aimed to perform high-resolution, full-range pH profiling within organoids.
“The Unisense microelectrodes and profiling system gave me the unique and exciting opportunity to interact with organoids - my favorite model system - with my own two hands. Automation is great, but the heterogeneity of organoid culture sometimes demands a more hands-on approach.”
Dr. Katrina Lyon, Dept Microbiology and Cell Biology, Montana State University
Laboratory Setup
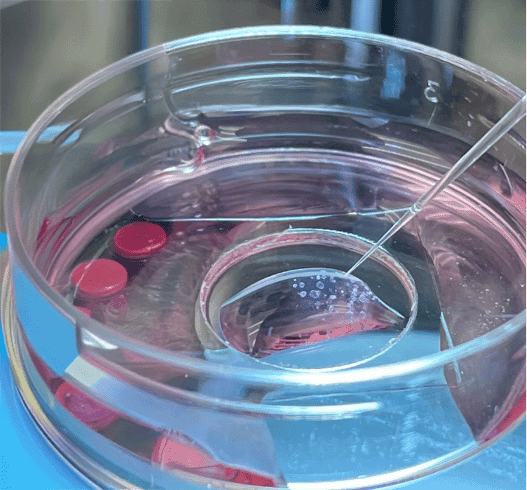
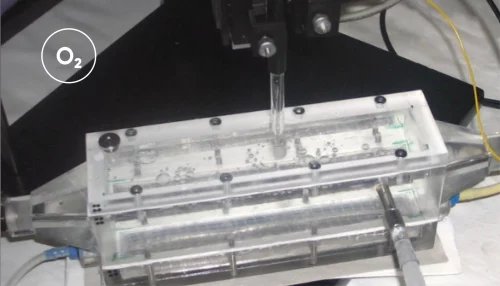
To establish the organoids, Lyon et al. obtained tissue samples from patients undergoing upper endoscopy and isolated the gastric glands. The glands were embedded in an extracellular matrix to support organoid formation in 3D culture. After several passages, the organoid cultures were established in glass-bottom dishes containing culture media. These were positioned on a stereomicroscope stage with a surrounding Unisense MicroProfiling System (Figure 1).
A Unisense pH microelectrode with a 25 μm tip was mounted in a micromanipulator, while a reference electrode was placed in the medium. The microelectrode was customized with a beveled tip to minimize damage to the organoids. Under continuous visual guidance through the stereomicroscope, the pH microelectrode was carefully advanced into the organoid lumen, to ensure precise insertion. pH measurements were taken both in the culture medium and across spatial gradients from the extracellular matrix to the organoid interior.

Results and Conclusion
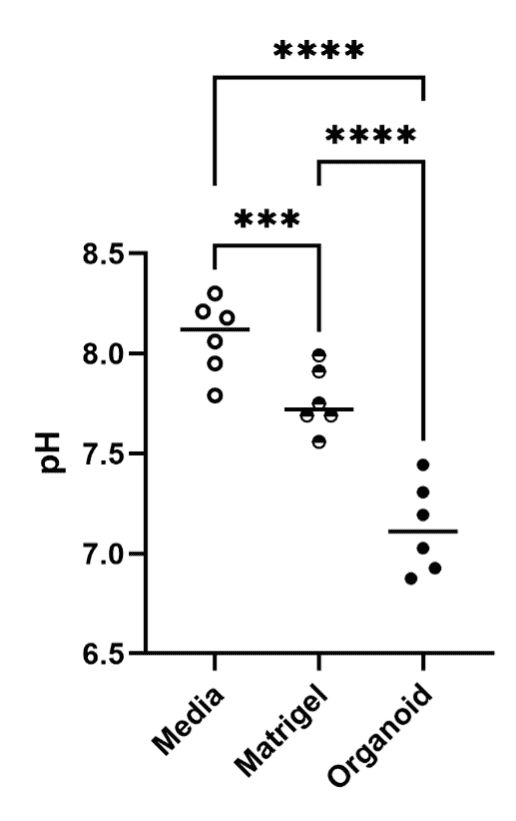
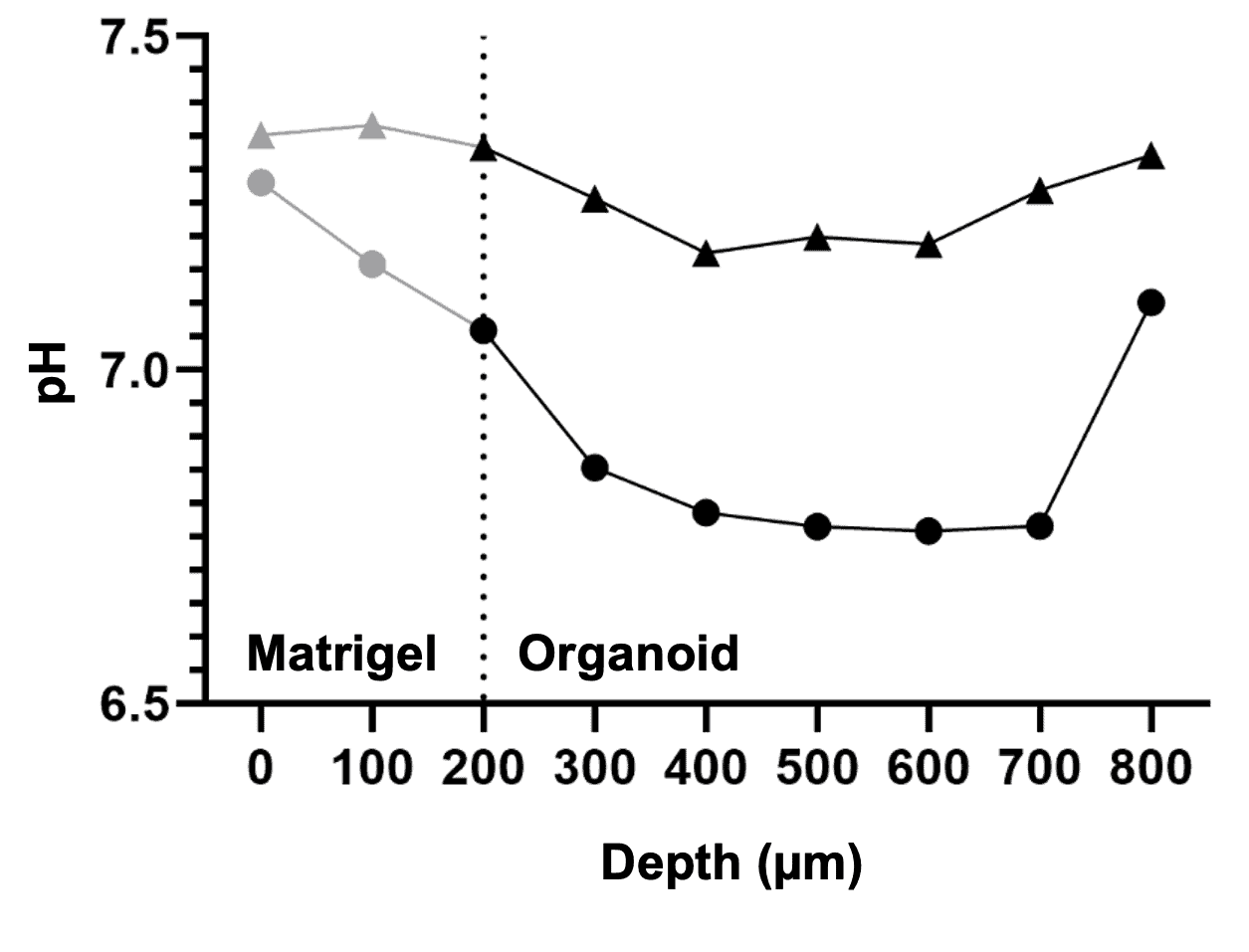
The internal pH of the organoids was significantly lower than that of the surrounding matrix and culture medium (Figure 2). This indicates that the organoids can maintain distinct internal environments, underscoring their physiological relevance. Spatial profiling further showed a marked pH drop from the interface between the extracellular matrix and the epithelium toward the center of the organoid lumen (Figure 3).
In their experimental protocol, Lyon et al. described the use of pH microelectrodes to obtain pH measurements with high spatiotemporal resolution. The authors validated the use of these microelectrodes in a gastrointestinal organoid model and concluded that they represent a valuable complement to conventional pH measurement methods. Finally, the approach described by Lyon et al. may be adapted for the measurement of other analytes in organoid systems.
You can read more in the article by Lyon et al. “Profiling Luminal pH in Three-Dimensional Gastrointestinal Organoids Using Microelectrodes”, Journal of Visualized Experiments (JoVE) 209 (2024): e66900.
Related Publications
Suggested Products

UniAmp Multi Channel for all Unisense sensors and electrodes including optical sensors
Microprofiles with extreme accuracy, high spatial and temporal resolution

Economic amplifier portfolio for single analytes - O2, pH/mV, H2, N2O or H2S
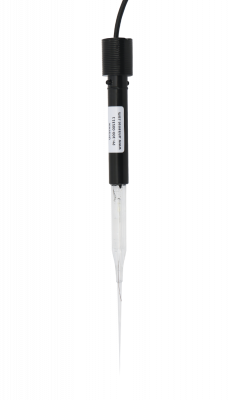
Miniaturized pH electrode

Reference electrode for pH or Redox microelectrodes
Automated or manual profiling, data visualization, and activity rate calculations.
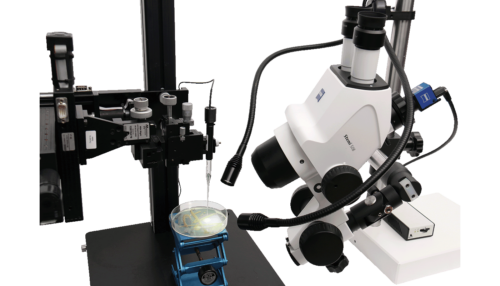
Keep an eye your sensor's movements with this customized Zeiss microscope
Application Notes

Bang et al. developed a dual flow chamber that mimics the shear stress and anaerobic conditions of the colon

Quantifying the Hydrogen Evolution Reaction using H2 Microsensors

Real-time measurements of oxygen consumption rates in response to 2-deoxy-D-glucose treatment

pH Microelectrode reveals distinct environment inside 3D organoid
Tissue oxygen partial pressure for screening and controlling for variation in ex vivo acute brain slice viability

How nitrifying microorganisms are able to produce nitrous oxide through denitrification

Hydrogen and oxygen microsensors for direct and real-time detection of dissolved gas in photocatalytic/electrochemical studies

The Eddy Covariance system and how to get high quality data from long term deployments with multiple sensors.

Learn in the laboratory - explore and confirm in the field!

Leaf gas films are hypothesized to improve internal aeration of the plant during the day

How to quantify the consumption rate of oxygen as well as the oxygen exchange rate across the water - sediment interface

Mitigation of N2O Emissions from Wastewater Biofilms
The use of oxygen microelectrodes to study nitritation biofilms with different geometries

O2 and N2O microprofiles in sputum samples from cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection
Oxygen and Redox Potential in Pseudomonas Aeruginosa Colony Biofilms
