

An anaerobic gastrointestinal host-microbe flow model
Measuring oxygen levels inline using optodes
Introduction
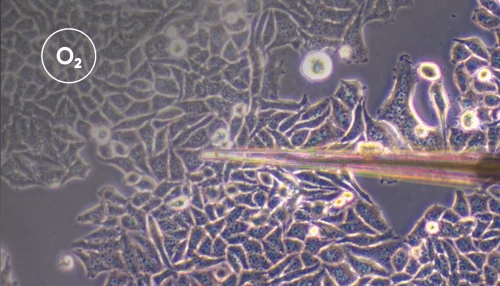
Understanding interactions between the human gut epithelium and anaerobic bacteria remains a central challenge in microbiology and infection research. Traditional in vitro cell culture models lack the complexity of the gut environment, while animal models do not fully replicate human susceptibility to intestinal pathogens, such as Clostridioides difficile. This has left a need for robust and accessible models that can mimic both the physiological shear stress and the anaerobic environment of the colon.
Maintaining stable, anaerobic conditions mimicking the human gut is central to the success of such a model. Existing models have utilized materials that are highly oxygen permeable, which compromises the models’ compatibility with microorganisms that require strict anaerobic conditions.
To address this challenge, Bang et al. developed a dual flow chamber model that supports long-term co-culture of intestinal epithelial cells with obligate anaerobic bacteria under controlled conditions.
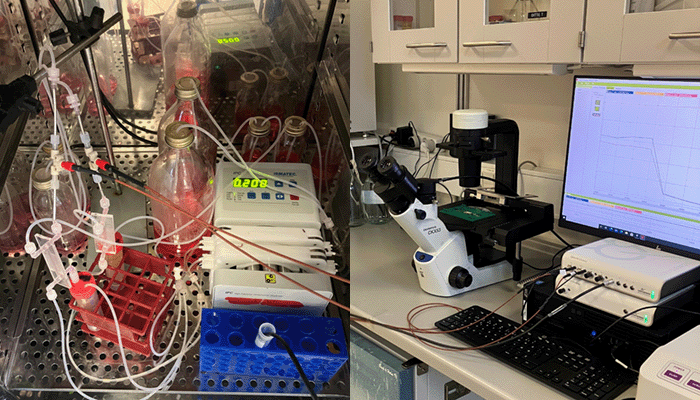
Laboratory Setup
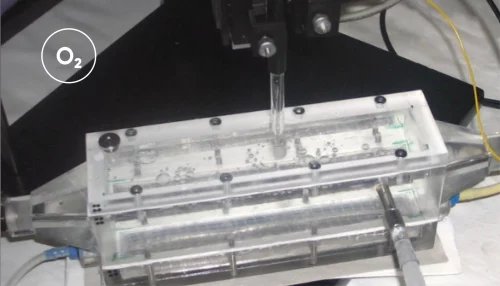
To ensure sufficient oxygen supply to the epithelial cells while maintaining strict anaerobic conditions for the bacteria, the team implemented a flow-based dual chamber design integrated with an anaerobization unit (Figure 1). In this setup, culture medium was supplied to both the apical and basal sides of the chamber, with the apical medium being continuously deoxygenated before entering the channel to maintain oxygen levels below 1%. Because the basal medium remained oxygenated, epithelial viability was not compromised.
Inline monitoring of oxygen levels was performed using a Unisense oxygen optode mounted in a PEEK flow-through cell. This enabled real-time verification of oxygen depletion efficiency and stability throughout the experiments, ensuring that the model accurately reproduced the low-oxygen environment of the human gut.

“Over the past few years, we have used the MicroOptodes from Unisense to validate the anaerobic conditions in our infection models. We appreciate the user-friendliness of both the sensors and software from Unisense, and Unisense has been very helpful in customizing the sensors to meet our specific requirements.”
PhD candidate Line Lundegård Bang, Department of Clinical Research, Odense University Hospital
Results and conclusion
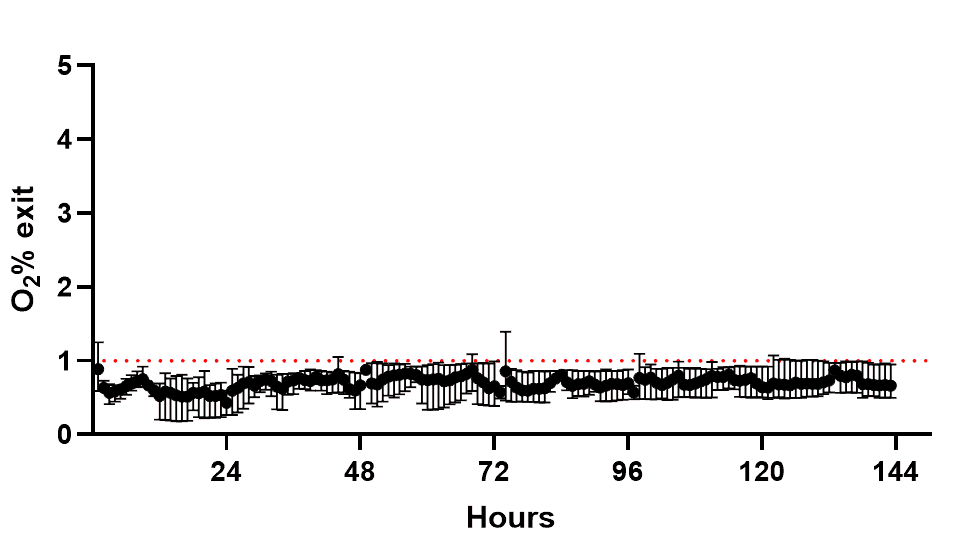
Initial validation of the system confirmed that anaerobic conditions could be reliably maintained. Oxygen measurements from the dual flow chambers with mature epithelial cells demonstrated stable anaerobic levels between 0.1–1% over six days (Figure 2), confirming that the setup provided a consistent low-oxygen environment.
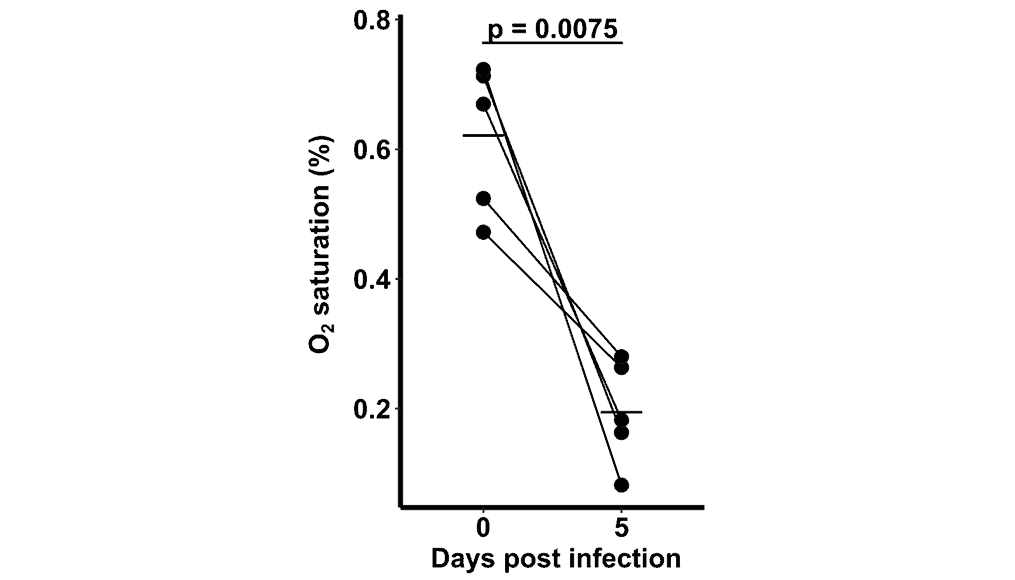
When the model was subsequently colonized with C. difficile, oxygen levels remained low despite bacterial growth and the barrier properties against oxygen diffusion were preserved, indicating an uncompromised epithelium even under infection conditions (Figure 3). Importantly, the model reflected a clinically relevant phenotype: persistence of C. difficile following vancomycin treatment, mirroring the antibiotic tolerance observed in patients.
Inline measurements with the Unisense oxygen optode were central to these insights, as they enabled continuous real-time monitoring of oxygen levels without disturbing the system.
You can read more in the article by Bang et al. “An anaerobic in vitro flow model for studying interactions at the gastrointestinal host-microbe interface”, npj Biofilms and Microbiomes, 2025, 11:160
Related Publications
Suggested Products

UniAmp Multi Channel for all Unisense sensors and electrodes including optical sensors

Optical oxygen sensor technology

Economic amplifier portfolio for single analytes - O2, pH/mV, H2, N2O or H2S

Monitor temperature in your sample
Calibrate your sensors and log time-series data.
Application Notes

Bang et al. developed a dual flow chamber that mimics the shear stress and anaerobic conditions of the colon

Quantifying the Hydrogen Evolution Reaction using H2 Microsensors

Real-time measurements of oxygen consumption rates in response to 2-deoxy-D-glucose treatment

pH Microelectrode reveals distinct environment inside 3D organoid
Tissue oxygen partial pressure for screening and controlling for variation in ex vivo acute brain slice viability

How nitrifying microorganisms are able to produce nitrous oxide through denitrification

Hydrogen and oxygen microsensors for direct and real-time detection of dissolved gas in photocatalytic/electrochemical studies

The Eddy Covariance system and how to get high quality data from long term deployments with multiple sensors.

Learn in the laboratory - explore and confirm in the field!

Leaf gas films are hypothesized to improve internal aeration of the plant during the day

How to quantify the consumption rate of oxygen as well as the oxygen exchange rate across the water - sediment interface

Mitigation of N2O Emissions from Wastewater Biofilms
The use of oxygen microelectrodes to study nitritation biofilms with different geometries

O2 and N2O microprofiles in sputum samples from cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection
Oxygen and Redox Potential in Pseudomonas Aeruginosa Colony Biofilms
