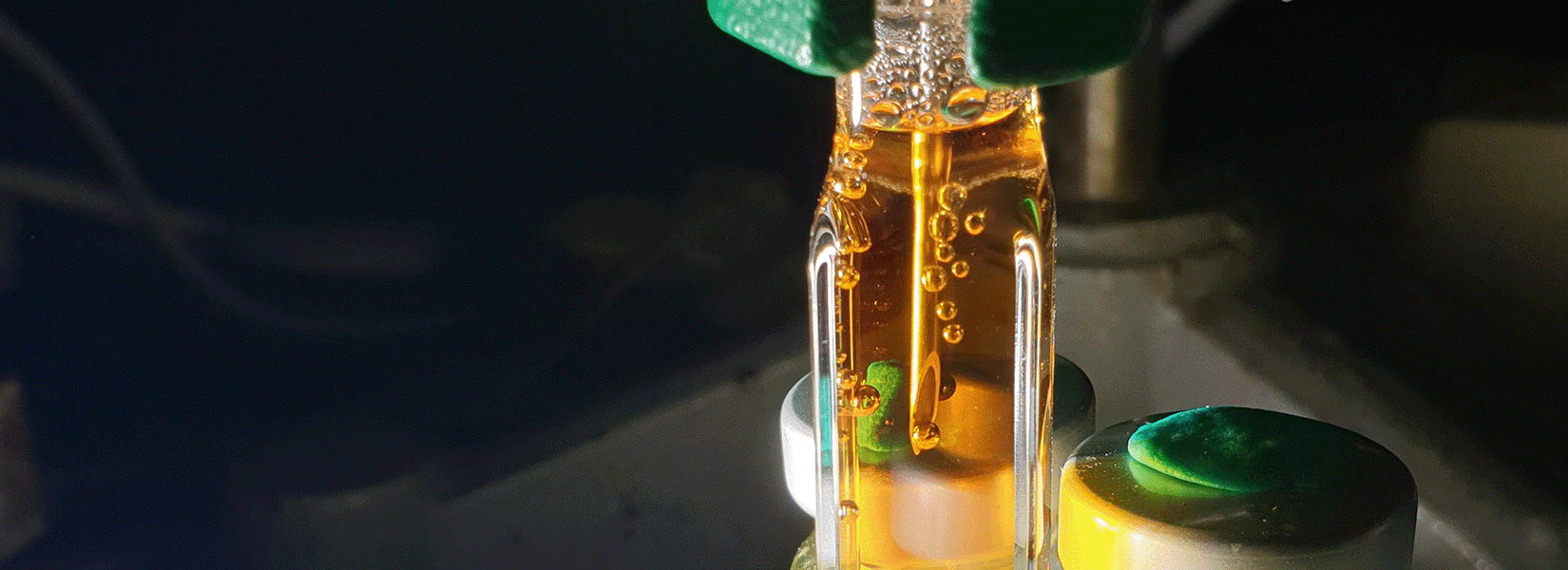

Hydrogen Production by a Fully De Novo Enzyme
Introduction
Hydrogen utilization is a promising alternative to fossil fuels where molecular, metal-based catalysts represent a sustainable opportunity. However, many molecular catalysts require rare metals or organic solvents, reducing their sustainable potential.
Cobaloxime is a molecular catalyst containing the globally available metal, cobalt, but its low solubility and stability has hindered its broader application. In a study from 2024, Berglund et al. sought to address this challenge by designing a de novo artificial enzyme for hydrogen production.
The researchers engineered a 65-amino acid protein consisting of three alpha-helices and covalently linked cobaloxime to a cysteine residue. This would effectively solubilize cobaloxime, but also partially bury it within the protein scaffold, potentially reducing the efficacy of hydrogen production from the artificial enzyme compared with free cobaloxime.

“The Unisense H2 Sensors have been massively useful in my research. Being able to continuously monitor the hydrogen evolution directly in solution gives me immediate insight into the behaviour of my catalysts. The sensors and the software are easy to use, and when I have had any questions or issues arise they have always been quickly resolved by their staff.”
PhD candidate Sigrid Berglund, Department of Chemistry, Uppsala University
Laboratory Setup
The researchers quantified the Hydrogen Evolution Reaction (HER) of the artificial enzyme and compared it to free cobaloxime. Both catalysts were placed in sealed vials with septum closures to prevent gas exchange with the atmosphere, and hydrogen production was initiated either photocatalytically or chemically.
For the photocatalytic HER, the artificial enzyme and free cobaloxime were illuminated in the presence of [Ru(bpy)3]2+ and ascorbic acid as an electron donor.
A Hydrogen Sensor in a piercing needle (H2-NPLR) was inserted through the septum and submerged in the solution (Figure 1). Once HER was triggered, the sensor continuously monitored hydrogen levels in real time.

Results and Conclusion
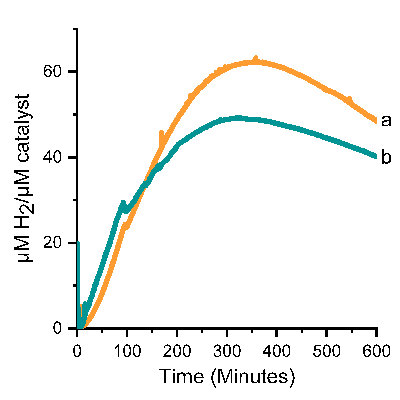
Under photocatalytic conditions, the artificial enzyme exhibited a rapid initial increase in hydrogen production, reaching a peak concentration of approximately 49 μM per μM catalyst (Figure 2). Free cobaloxime showed a similar trend but achieved a higher maximum hydrogen concentration of 62 μM per μM catalyst.
Consequently, the hydrogen production of the artificial enzyme corresponds to 80% of the hydrogen production of free cobaloxime, indicating a slightly reduced catalytic efficiency when cobaloxime was embedded in the protein scaffold. The researchers thereby demonstrated the potential of using artificial enzymes for hydrogen production.
The H2 Sensor enabled continuous, realtime measurements of dissolved hydrogen throughout the illumination period. Its piercing needle design allowed insertion into the sealed vials while avoiding gas exchange with the atmosphere.
You can read more in the article by Berglund et al. “Hydrogen production by a fully de novo enzyme”, Dalton Trans., 2024, 53,12905.
Related Publications
Suggested Products
High performance oxygen microsensor

UniAmp Multi Channel for all Unisense sensors and electrodes including optical sensors

Economic amplifier portfolio for single analytes - O2, pH/mV, H2, N2O or H2S
Measure dissolved and gaseous hydrogen with a high-precision

Monitor temperature in your sample
Calibrate your sensors and log time-series data.
Application Notes

Bang et al. developed a dual flow chamber that mimics the shear stress and anaerobic conditions of the colon

Quantifying the Hydrogen Evolution Reaction using H2 Microsensors

Real-time measurements of oxygen consumption rates in response to 2-deoxy-D-glucose treatment

pH Microelectrode reveals distinct environment inside 3D organoid
Tissue oxygen partial pressure for screening and controlling for variation in ex vivo acute brain slice viability

How nitrifying microorganisms are able to produce nitrous oxide through denitrification

Hydrogen and oxygen microsensors for direct and real-time detection of dissolved gas in photocatalytic/electrochemical studies

The Eddy Covariance system and how to get high quality data from long term deployments with multiple sensors.

Learn in the laboratory - explore and confirm in the field!

Leaf gas films are hypothesized to improve internal aeration of the plant during the day

How to quantify the consumption rate of oxygen as well as the oxygen exchange rate across the water - sediment interface

Mitigation of N2O Emissions from Wastewater Biofilms
The use of oxygen microelectrodes to study nitritation biofilms with different geometries

O2 and N2O microprofiles in sputum samples from cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection
Oxygen and Redox Potential in Pseudomonas Aeruginosa Colony Biofilms
