
Water Splitting Studies
Hydrogen production at the surface of a semiconductor material
Photoelectrochemical water splitting is a promising route to sustainable hydrogen production, but progress is often limited by the performance of photoabsorber materials. To explore new options, Jonathan Kampmann and coworkers at the University of Munich investigated covalent organic frameworks (COFs) as a novel class of photoelectrodes.
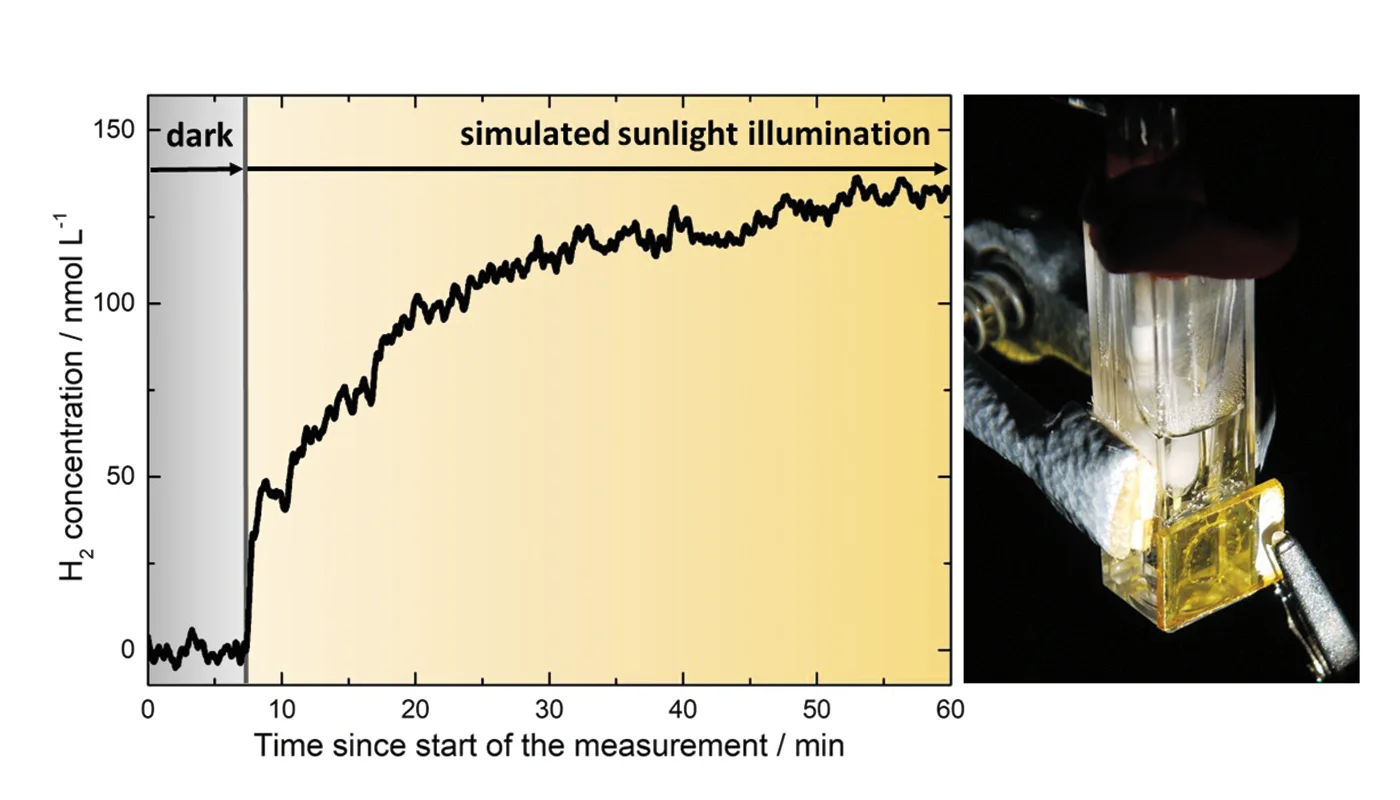
Using a low range Hydrogen Sensor in a piercing needle (H2-NP), the team detected nanomolar hydrogen concentrations evolving directly in solution at the COF surface under light illumination (Figure 1).
The sensor enabled continuous, sensitive detection of hydrogen formation, confirming the photoelectrochemical activity and stability of the oriented COF films. These findings demonstrate COFs as promising photoelectrodes for water splitting.
Read more in: Sick et al., J. Am. Chem. Soc. 2018, 140, 2085–2092.
Data and photo kindly provided by Jonathan Kampmann
Hydrogen production catalyzed by organic polymer dots
Organic semiconductors have emerged as promising photocatalysts for light-driven hydrogen generation, offering metal-free structures, tunable optical properties, and material abundance. Dr. Haining Tian and his group at Uppsala University investigated polymer dots (Pdots) as photocatalysts for visible-light-driven water splitting.
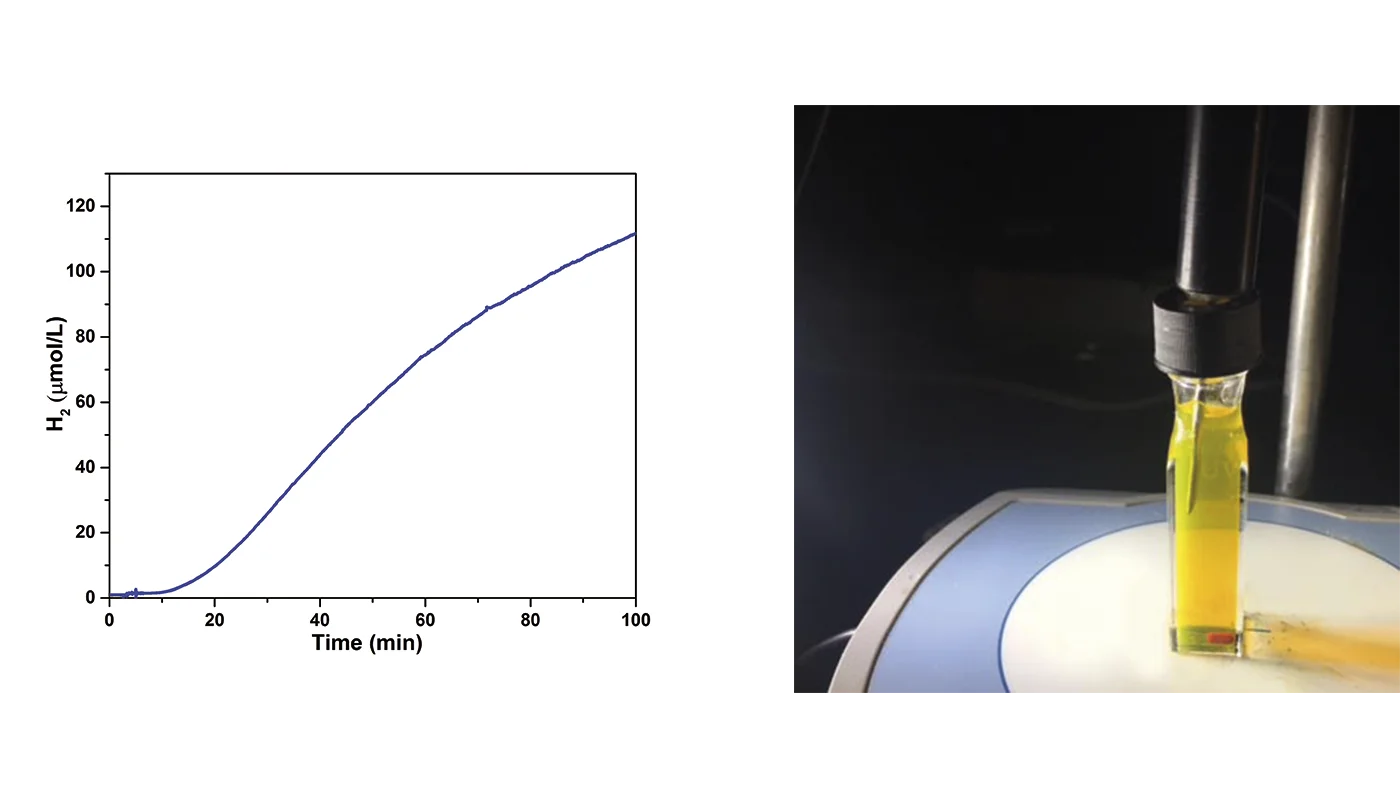
Using a Unisense H2-NP low range sensor with a detection limit of 50 nM, hydrogen evolution was monitored directly in aqueous Pdot suspensions by inserting the H2 sensor through the cuvette lid. Continuous and sensitive measurements confirmed that the Pdots efficiently catalyzed hydrogen production under visible light, achieving activity several orders of magnitude higher than pristine polymer material.
Importantly, the Pdots also demonstrated tolerance to oxygen, a key advantage for practical application. These results highlight Pdots as a highly effective class of photocatalysts for hydrogen generation.
Data and photo kindly provided by Dr. Haining Tian.
For further reading please see the article: Wang et al. Organic Polymer Dots as Photocatalysts for Visible Light-Driven Hydrogen Generation. Angew. Chem. Int. Ed. 2016.
Related Publications
Related products
High performance oxygen microsensor

UniAmp Multi Channel for all Unisense sensors and electrodes including optical sensors

Economic amplifier portfolio for single analytes - O2, pH/mV, H2, N2O or H2S
Measure dissolved and gaseous hydrogen with a high-precision
Calibrate your sensors and log time-series data.
