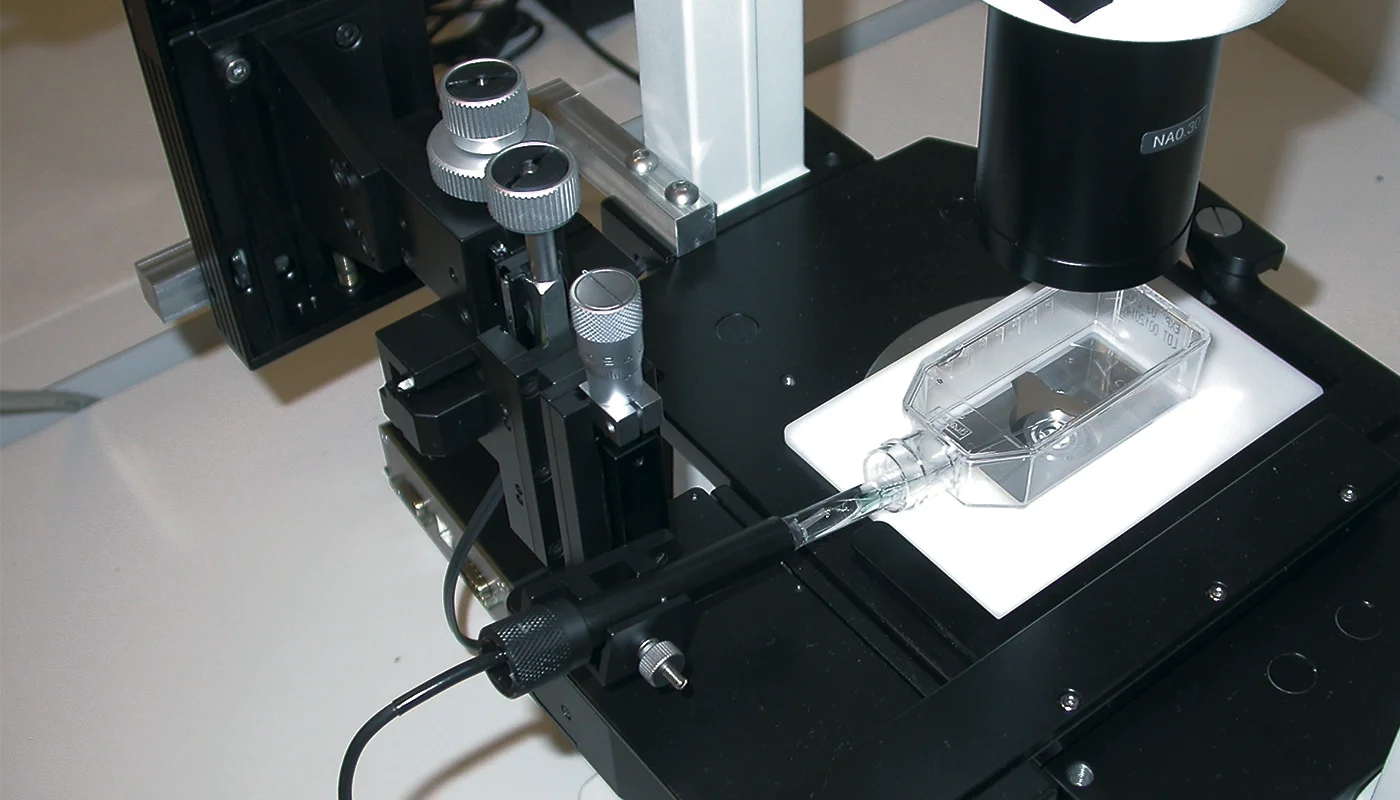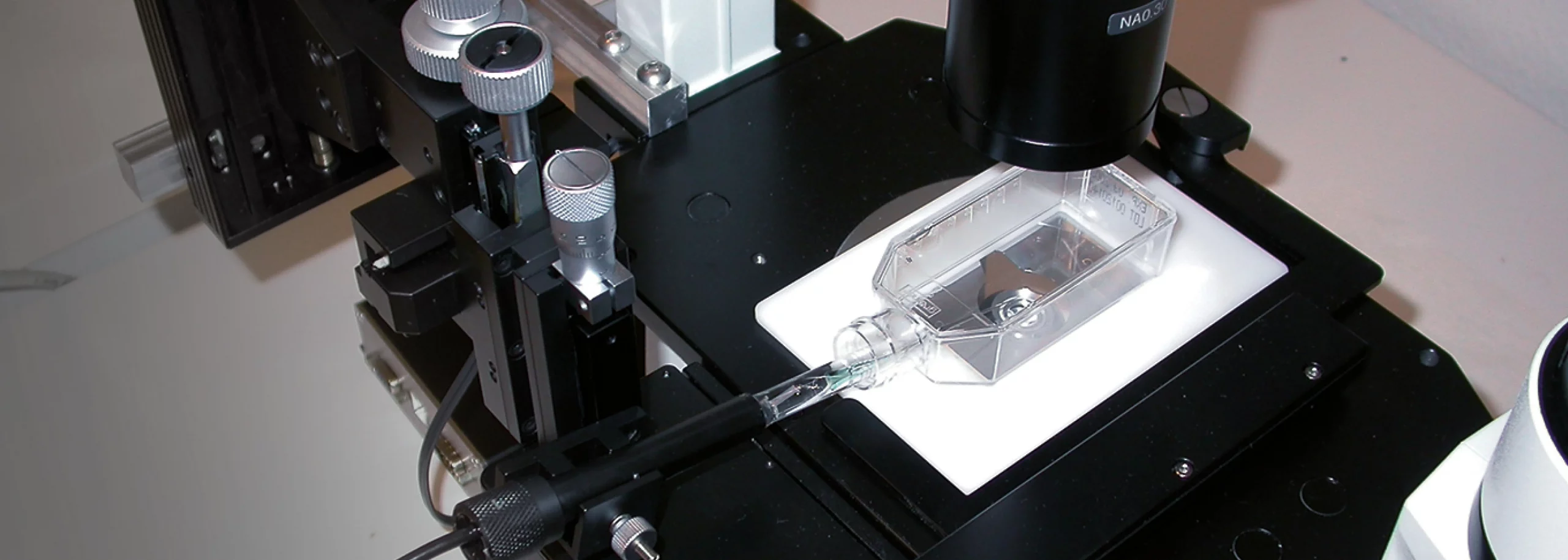
Cell Culture Profiling
The respiration of a cell culture combined with the low diffusion rate of oxygen in the growth medium can lead to a marked decrease in oxygen tension close to the cells. Depending on the particular circumstances, this phenomenon may interfere with other factors that you attempt to study and complicate interpretation of the results. Unisense developed a set-up for the detailed study of pericellular oxygen concentration and consumption rates of an adhering cell culture (Pettersen et.al. 2005).
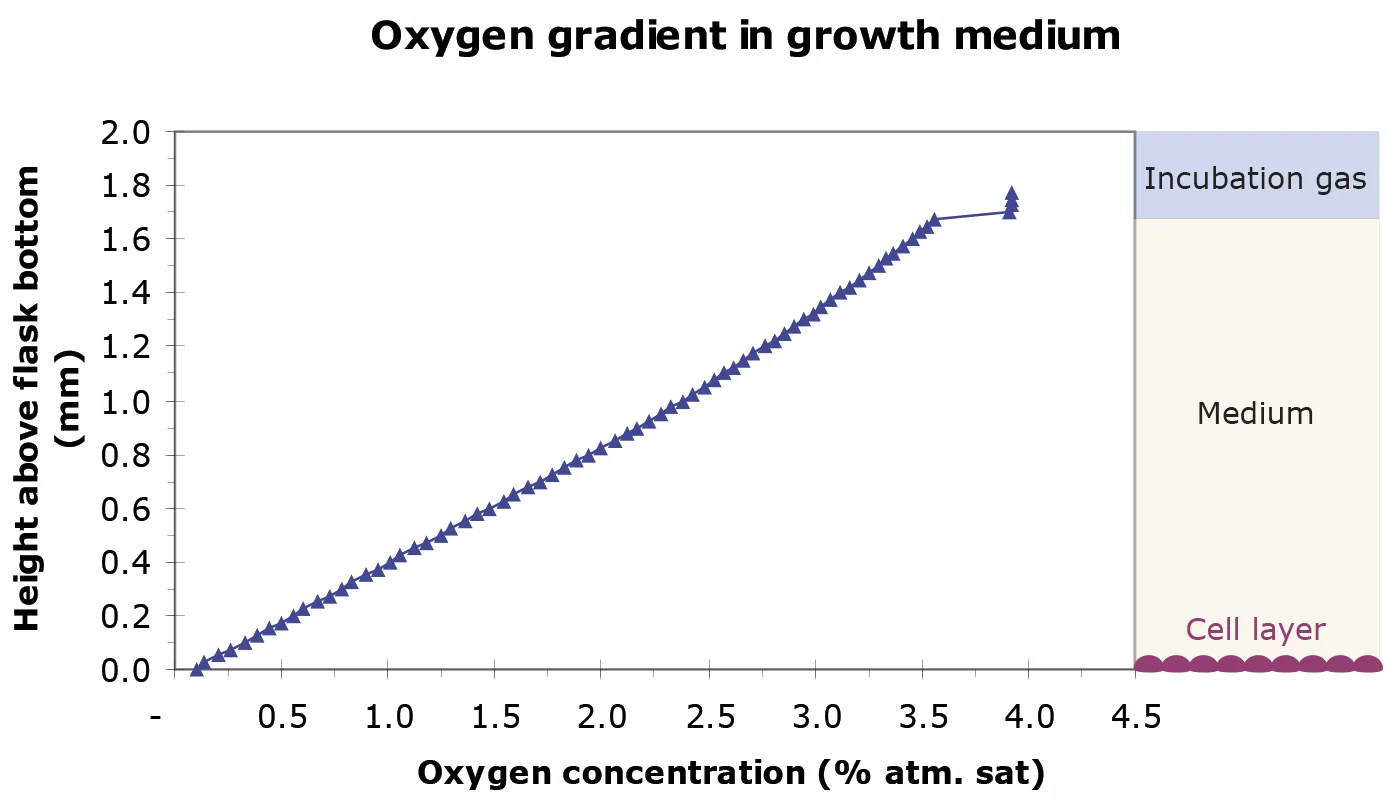
Using this set-up, the oxygen consumption of a carcinoma cell monolayer was studied. As seen in the graph above, the measurements showed a highly reduced pericellular oxygen concentration. Not only the pericellular concentration, but also oxygen gradients through the growth medium can be measured, allowing consumption rates to be calculated directly from the provided data acquisition software utility. In this setup, the oxygen utilization rate is 360 fmol O2/cell/h.
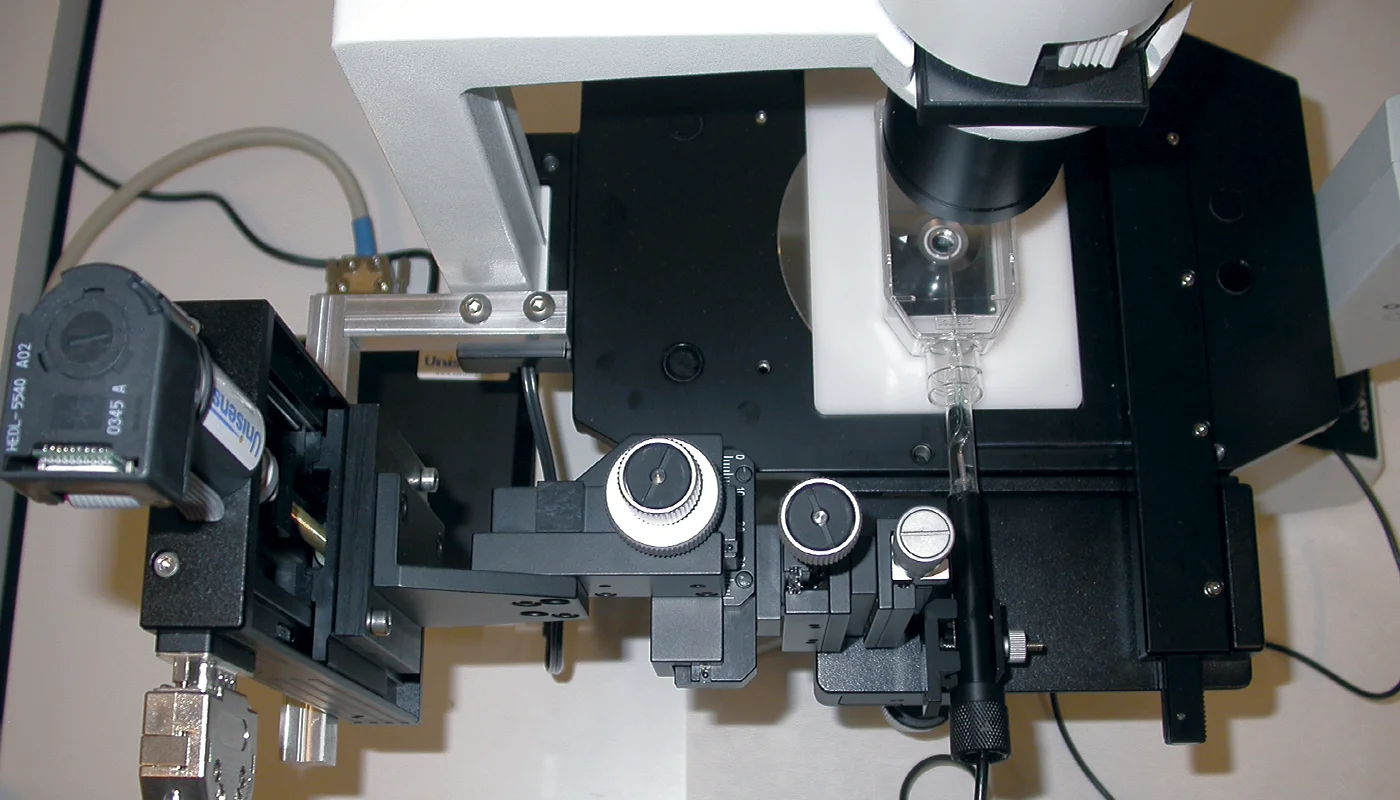
The setup was mounted on an inverted microscope, which allows the user to observe when the sensor tip touches the flask bottom. The sensor is controlled by a motorized micromanipulator and tilted at an angle, allowing the sensor to flex slightly and not break when touching the flask bottom.
The cells are cervical carcinoma cells grown in a 4% O2 atmosphere. During the first 15 days, the cells are passaged twice a week. After seeding on day 15, the medium is changed on day 17 and 19 and the profile and picture shown here is taken on day 20.